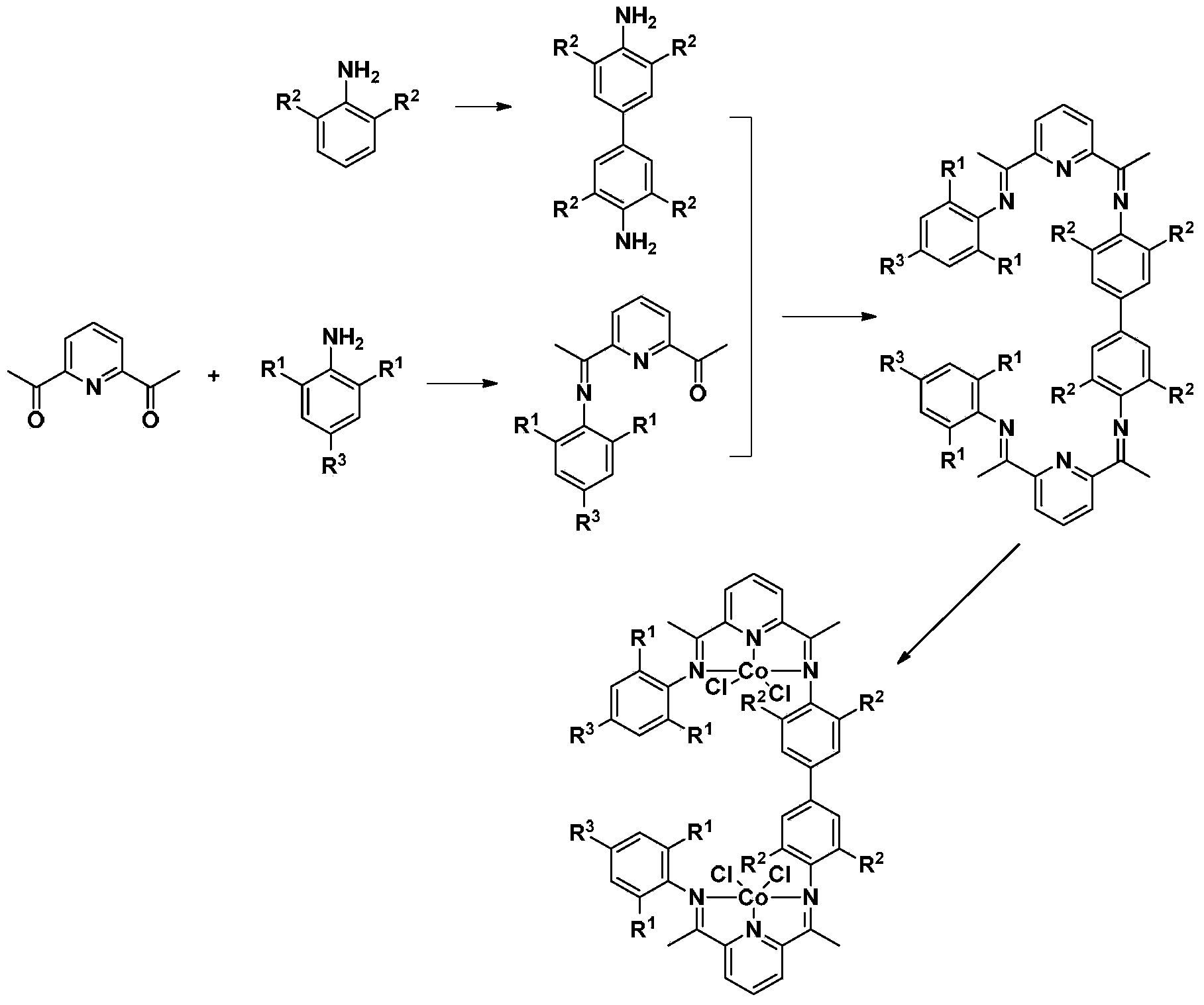
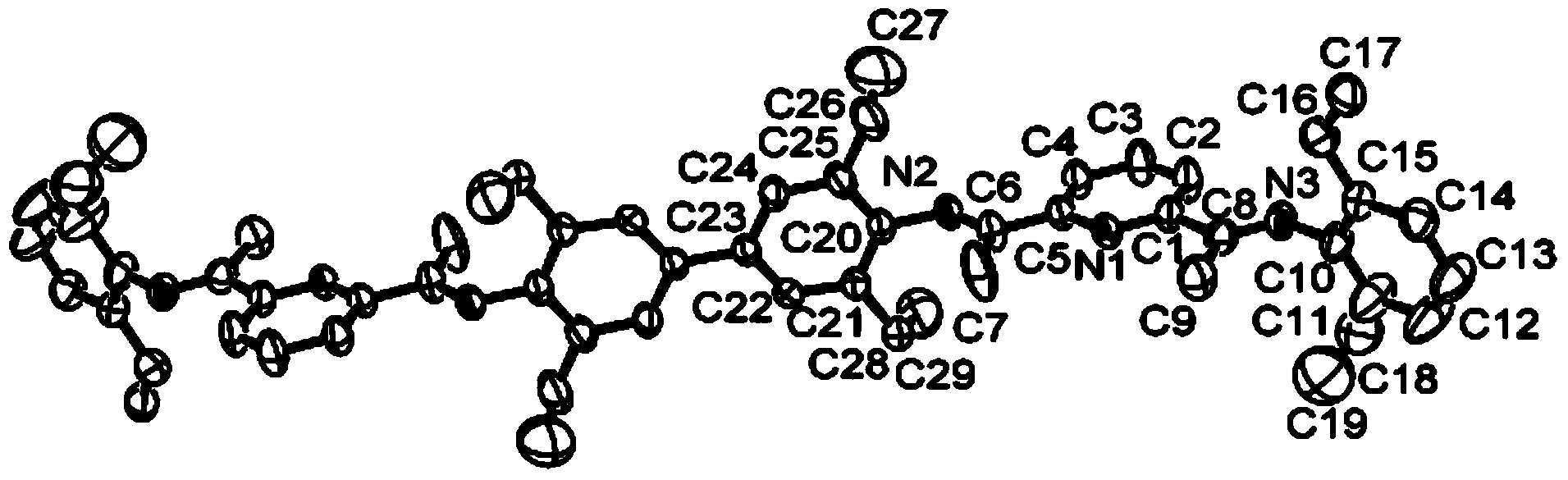
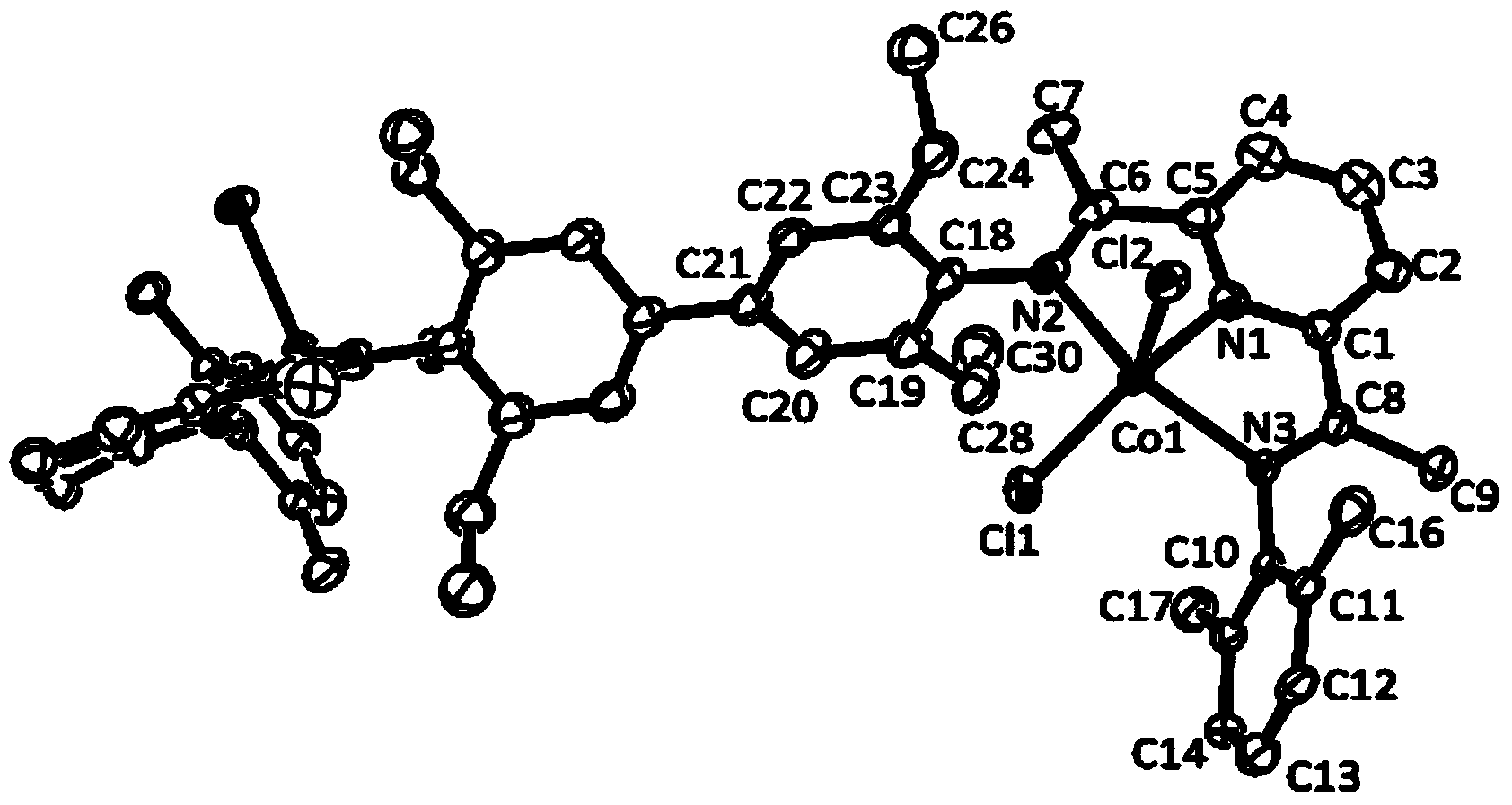
2,6-diene amine pyridine binuclear cobalt complex catalyst as well as preparation method and application thereof
A technology of dienylaminopyridine and cobalt complexes, which is applied in the production of cobalt organic compounds, organic chemistry, bulk chemicals, etc., and can solve problems such as limited metal catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1, preparation N, N / - Two (1-(3-(1-(2,6-diethylphenylimine) ethyl) pyridin-2-yl) ethylene) tetraethylbiphenyl (L5) (R 1 is ethyl, R 2 for hydrogen, R 3 for ethyl)
[0090]
[0091] 1) Add catalyst amount (2ml) to a solution of 2,6-diacetylpyridine (3.9g, 24mmol) and 2,6-diethylaniline (3.0g, 20mmol) of formula II in ethanol (200mL) Formic acid, stirred in an ice bath for 24h, filtered to obtain 0.87g yellow solid, belonging to formula III (R 1 is ethyl, R 2 2-enamine-6-acylpyridine compound for hydrogen): 1-(6-(1-((2,6-diethylphenyl)enamine)ethyl)2-pyridyl)acetyl, yield 29.5 %.
[0092]2) Dissolve 6.0g (40mmol) 2,6-diethylaniline in 50ml dichloromethane, 20g (133mmol) copper sulfate and 20g (126mmol) potassium permanganate are fully ground and mixed, add to the solution and stir at room temperature for 15h. Reaction solution The completion of the reaction was confirmed by thin-layer chromatography, and the reaction solution was subjected to column...
Embodiment 2
[0098] Embodiment 2, preparation N,N / -Bis(1-(3-(1-(2,6-dimethylphenylimine)ethyl)pyridin-2-yl)ethylene)tetramethyl Biphenyl[L1] (R 1 is methyl, R 2 for hydrogen, R 3 for methyl)
[0099]
[0100] Using the same method as in Example 1, 0.45 g of a yellow solid was obtained with a yield of 49.3% (melting point: 272° C.).
[0101] The structural confirmation data are as follows: 1 H NMR: (400MHz, CDCl 3 ):8.53(d,J=8.0Hz,2H,Py-H),7.96(t,J=8.0Hz,1H,Py-H),7.37(d,J=8.0Hz,2H,Ph-H), 7.09(t, J=8.0Hz, 1H, Ph-H), 6.96(s, 2H, Ph-H), 2.31(s, 3H, CH 3 ),2.26(s,3H,CH 3 ),2.14(s,6H,2×CH 3 ),2.07(s,6H,2×CH 3 ). 13 C NMR (100MHz, CDCl 3 ):167.5,167.3,155.2,155.1,148.8,148.7,136.9,136.0,128.0,126.4,125.8,125.5,123.1,122.4,18.2,18.0,16.7,16.5.FT-IR(cm -1 ):2913(m),1639(ν C=N ,s),1569(m),1461(s),1429(s),1362(s),1324(w),1296(w),1247(m),1200(s),1120(m),1083 (m), 857(m), 814(m), 769(s), 689(w). Elemental analysis: C 50 h 52 N 6 (736) Theoretical value: N, 11.40; C, 81.49; H...
Embodiment 3
[0102] Embodiment 3, preparation N, N / - Two (1-(3-(1-(2,6-diethylphenylimine) ethyl) pyridin-2-yl) ethylene) tetramethylbiphenyl [L2] (R 1 is ethyl, R 2 for hydrogen, R 3 for methyl)
[0103]
[0104] Using the same method as in Example 1, 0.41 g of a yellow solid was obtained with a yield of 42.6% (melting point: 238° C.).
[0105] The structural confirmation data are as follows: 1 H NMR (400MHz, CDCl 3 ):8.51(d,J=8.0Hz,4H,Py-H),7.95(t,J=8.0Hz,2H,Py-H),7.13(d,J=7.6Hz,4H,Ph-H), 7.05(t, J=8.0Hz, 2H, Ph-H), 6.29(s, 4H, Ph-H), 2.29-2.44(m, 8H, 4×CH 2 ),2.24(s,6H,2×CH 3 ),2.10(s,6H,2×CH 3 ),1.14(t,J=7.6Hz,12H,4×CH 3 ). 13 C NMR (100MHz, CDCl 3 ):167.5,166.9,155.2,155.0,147.9,136.9,136.0,131.2,126.4,126.0,125.8,123.4,122.3,24.7,18.2,16.9,16.7,13.8.FT-IR(cm -1 ):2964(m),1637(ν C=N ,s),1567(s),1450(m),1362(s),1321(w),1296(w),1243(s),1200(m),1119(m),1098(w),857 (w), 828(m), 769(s), 689(w). Elemental analysis: C 56 h 60 N 6 (792) Theoretical value: N, 10.60; C, 81...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com