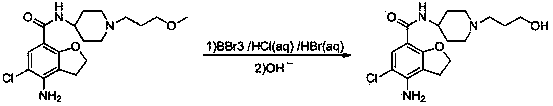Method for preparing prucalopride impurity
A technology of impurity and reaction, applied in the field of preparing prucalopride impurity, can solve the problem of high price of boron tribromide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Add 5.0 g of N-[1-(3-methoxypropyl)]-4-amino-5-chloro-2,3-dihydrobenzofuran-7-carboxamide into a 100 mL three-necked flask, and add 50 mL Hydrobromic acid (40%), heat up to 85°C to react, TLC detects the reaction, after the reaction is completed, cool down to room temperature, adjust the reaction solution to neutral with sodium hydroxide solution, filter the resulting solid, and dichloromethane and methanol recrystallized, N-[1-(3-hydroxypropyl)]-4-amino-5-chloro-2,3-dihydrobenzofuran-7-carboxamide, the yield was 75%.
Embodiment 2
[0015] Add 5.0 g of N-[1-(3-methoxypropyl)]-4-amino-5-chloro-2,3-dihydrobenzofuran-7-carboxamide into a 100 mL three-necked flask, add 30 mL Hydrobromic acid (40%), heat up to 70°C to react, TLC to detect the reaction, after the reaction is completed, cool down to room temperature, adjust the reaction solution to neutral with sodium hydroxide solution, filter the resulting solid, and dichloromethane and methanol recrystallized, N-[1-(3-hydroxypropyl)]-4-amino-5-chloro-2,3-dihydrobenzofuran-7-carboxamide, the yield was 55%.
Embodiment 3
[0017] Add 5.0 g of N-[1-(3-methoxypropyl)]-4-amino-5-chloro-2,3-dihydrobenzofuran-7-carboxamide into a 100 mL three-necked flask, add 30 mL Hydrobromic acid (40%), heat up to 100°C to react, TLC to detect the reaction, after the reaction is completed, cool down to room temperature, adjust the reaction solution to neutral with sodium hydroxide solution, filter the resulting solid, and dichloromethane and methanol recrystallized, N-[1-(3-hydroxypropyl)]-4-amino-5-chloro-2,3-dihydrobenzofuran-7-carboxamide, the yield was 70%.
[0018]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com