Demethylivabradine salt as well as preparation method and application thereof
A technology of sulfate and phosphate, which is applied in the field of medicine, can solve the problems affecting the production, quality, absorption and curative effect of medicines, and achieve the effect of favorable preparation and preservation, low requirements for storage environment and production equipment, and strong heart rate inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
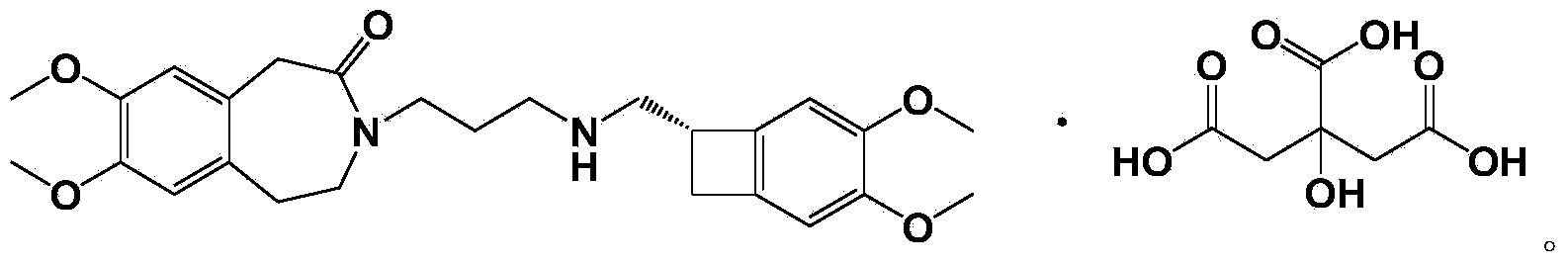
[0046] Example 1 Preparation of norivabradine citrate
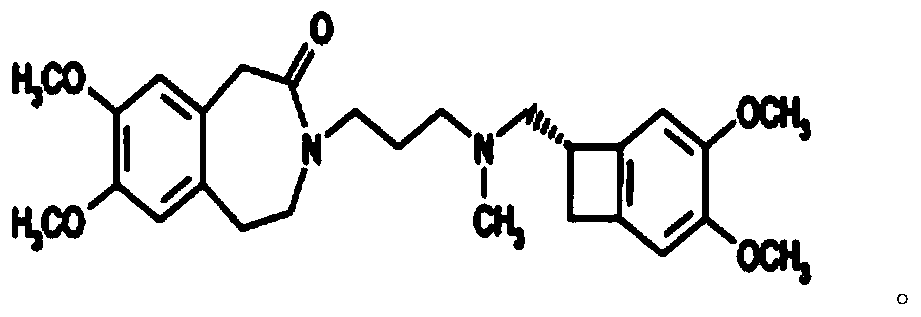
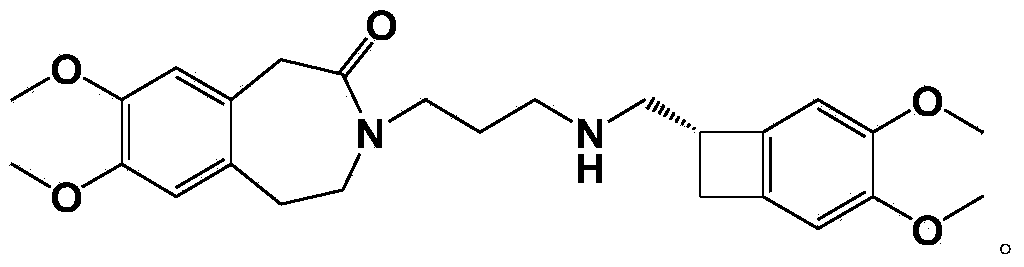
[0047] Prepare nor-ivabradine citrate by nor-ivabradine, the chemical reaction formula is as follows:
[0048]
[0049] 45.4 g (100 mmol) of norivabradine was dissolved in 1000 mL of acetone at 30° C., and 21.1 g (110 mmol) of citric acid were added. The mixture was stirred and reacted at room temperature for 2 hours, and the precipitated crystals were suction-filtered, rinsed with 200 mL of acetone, and vacuum-dried to obtain 52.4 g of norivabradine citrate, with a yield of 81%, HPLC: 99.734%.
[0050] The physicochemical properties and identification data of the prepared norivabradine citrate are as follows:
[0051] M.p.141-143℃:
[0052] DSC curve (closed pan 10°C / min): starting at 143.6°C.
[0053] IR spectrum (KBr, in cm -1 calculation): 417, 432, 460, 491, 516, 543, 570, 613, 639, 657, 677, 696, 723, 758, 789, 827, 860, 871, 885, 917, 937, 962, 980, 1005 . , 2644, 2833, 2921, 2935, 2969, 3384.
[0054] 1 H...
Embodiment 2
[0056] Example 2 Preparation of Norivabradine Citrate
[0057] Dissolve 4.54 g (10 mmol) of norivabradine in 100 mL of ethyl acetate at 30° C., and add 2.11 g (11 mmol) of citric acid. The mixture was stirred and reacted at room temperature for 2 hours, and the precipitated crystals were suction filtered, rinsed with 20 mL of ethyl acetate, and dried in vacuo to obtain 5.56 g of norivabradine citrate, with a yield of 86%, HPLC: 99.644% .
Embodiment 3
[0058] Example 3 Preparation of norivabradine citrate
[0059] 4.54 g (10 mmol) of norivabradine was dissolved in 100 mL of ethanol at 40° C., and 2.11 g (11 mmol) of citric acid were added. The mixture was stirred and reacted at room temperature for 2 hours, the temperature was raised to 55° C., 0.3 g of activated carbon was added for decolorization for 10 minutes, and filtered while hot. Place it at 20-25°C for crystallization for 3 hours. The precipitated crystals were suction-filtered, rinsed with 20 mL of ethanol, and vacuum-dried to obtain 5.36 g of norivabradine citrate, with a yield of 83%, HPLC: 99.840%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com