A virus nucleic acid extraction reagent
A virus nucleic acid and reagent technology, applied in the field of biotin-labeled specific probes, can solve the problems of magnetic bead coating, aerosol contamination, and long sample processing time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A viral nucleic acid extraction kit is provided in this embodiment, which contains a viral nucleic acid extraction reagent, and the reagent contains three biotin-labeled specific probes, namely biotin-labeled hepatitis B virus probe, biotinylated hepatitis C virus probe and biotinylated human immunodeficiency virus probe. Specifically, the kit includes the following components:
[0028] ① Magnetic beads containing specific probes: The magnetic beads containing specific probes include the following three types:
[0029] Magnetic beads containing HBV capture probe: magnetic beads-streptavidin-biotin-TTTTTTTTTTTTTT-TTGGGTGGCTTTGGGGCATGG;
[0030] Magnetic beads containing HCV capture probe: magnetic beads-streptavidin-biotin-TTTTTTTTTTTTTT-TGGTACTGCCTGATAGGGTGCTTGCG;
[0031] Magnetic beads containing HIV-I capture probe: magnetic beads-streptavidin-biotin-TTTTTTTTTTTTTT-CCAGGCCAGATGAGAGAACCAAGGG.
[0032] The streptavidin magnetic beads described in the present inventi...
Embodiment 2
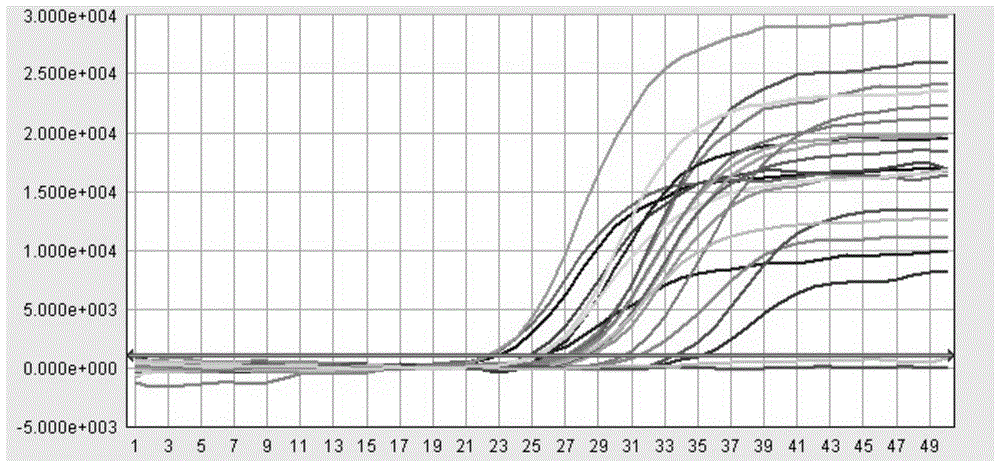
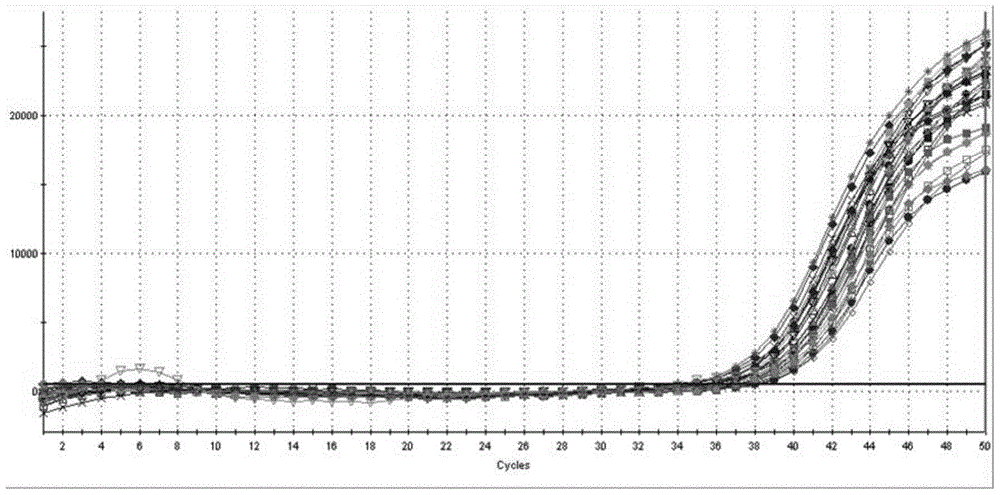
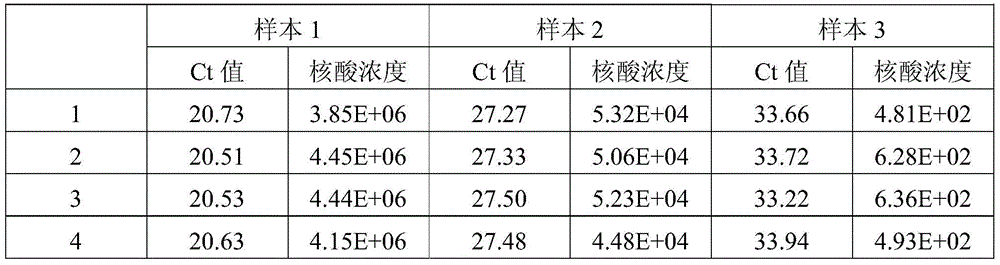
[0039] This example provides a method for extracting the HBV / HCV / HIV-I virus (HBV DNA in this example) in the sample to be tested using the kit described in Example 1, as well as the detection of the obtained viral nucleic acid by fluorescent quantitative PCR and its result.
[0040] First of all, the sample to be tested in the present invention is: the concentrations of the quantitative standards of hepatitis B virus (HBV) nucleic acid of the China Institute for the Control of Biological Products (National Institute for Inspection and Control) are 5.0×10 6 IU / ml, 5.0×10 4 IU / ml and 5.0×10 2 Three concentrations of HBV DNA positive serum in IU / ml.
[0041] The steps of nucleic acid extraction and detection are as follows:
[0042] 1) Cell lysis: add the sample to be tested into a centrifuge tube, add cell lysate to lyse the cells to release nucleic acids;
[0043] 2) Nucleic acid adsorption: Add magnetic beads containing specific probes to the solution, and the HBV / HCV / HIV...
Embodiment 3
[0053] This example provides a method for extracting the HBV / HCV / HIV-I virus (HCV RNA in this example) in the sample to be tested using the kit described in Example 1, as well as the detection of the obtained viral nucleic acid by fluorescent quantitative PCR and its result.
[0054] The samples to be tested in the present invention are: srea care HCV typing reference products diluted 10 times (20 kinds in total). In the present invention, two control systems (Abbott RealTime HCV m2000 detection system, Roche AmpliPrep / COBAS TaqManTest detection system) and the kit of the present invention are used to detect various subtypes of HCV in the serum of the test sample.
[0055] The extraction and detection method of the present invention: add 200 μl of the sample to be tested into a 1.5ml centrifuge tube, and then add 600 μl of RNA extraction solution 1 (a mixture of cell lysate and magnetic beads containing specific probes): the RNA extraction Solution 1 was composed of 0.5% (W / V...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com