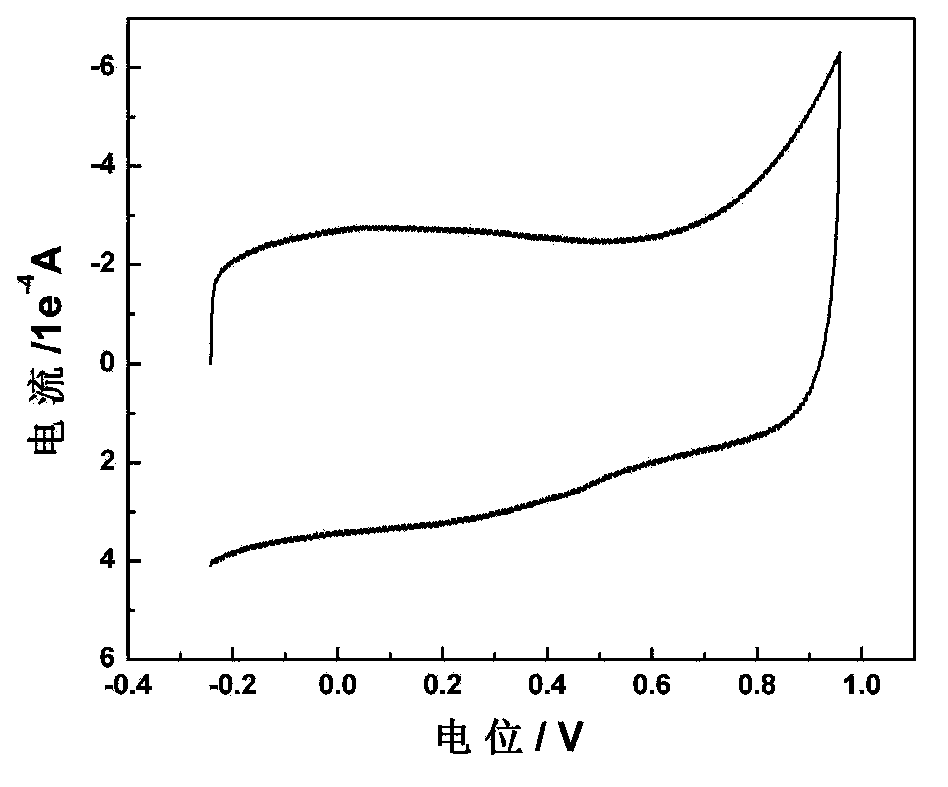
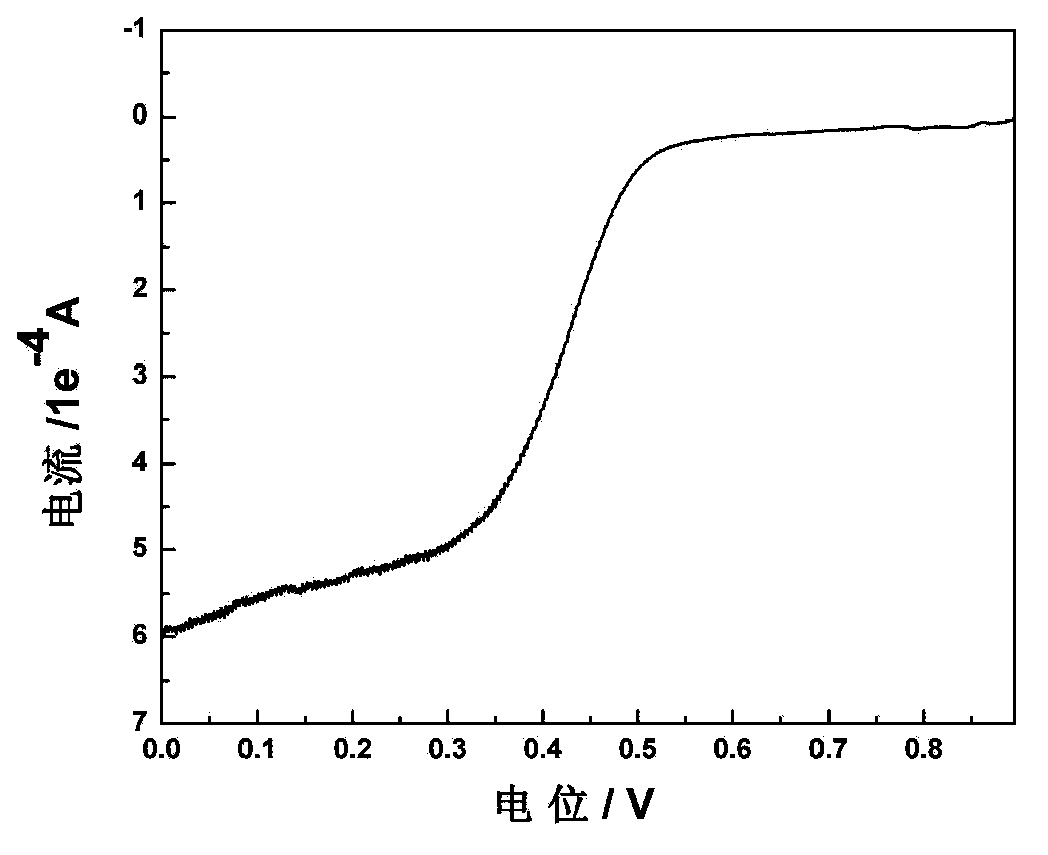
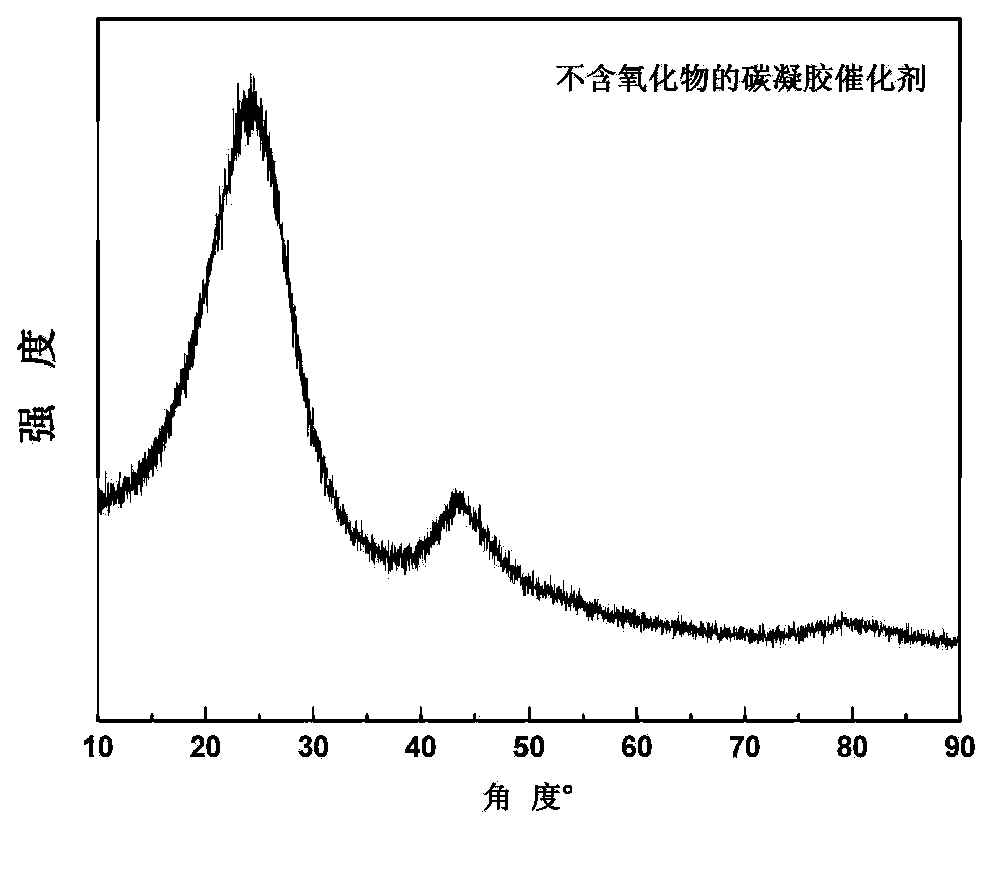
Carbon gel catalyst for fuel batteries and application thereof
A technology of fuel cells and catalysts, applied in the direction of physical/chemical process catalysts, battery electrodes, circuits, etc., can solve the problems of poor stability and low activity, and achieve the effects of improving stability, high catalytic activity, and improving uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Dissolve 6.16g of resorcinol in 7mL of deionized water to form a transparent solution A; take 0.815g of cobalt nitrate hexahydrate solid and add it to the above transparent solution A while stirring, and mix well to obtain solution B; 9.08g of formaldehyde solution with a mass concentration of 37% was stirred for 5h to prepare a sol-gel precursor. Take 2.128ml of zirconium oxynitrate solution of 20mg / ml (based on the mass of metal zirconium), add 1.5M dilute ammonia solution drop by drop in a 60°C water bath and stir to adjust the pH of the zirconium oxynitrate / ammonia water complex to 7 . The prepared zirconium oxynitrate / ammonia water complex was added dropwise to the sol-gel precursor under vigorous stirring to obtain a homogeneous mixture C, and 3 mL of ammonia water with a mass concentration of 28% was added dropwise in an environment of 20 ° C and continued to seal and stir, and the reaction Gel D was formed; the gel D was transferred to a vacuum drying oven for ...
Embodiment 2
[0051] Dissolve 6.16g of resorcinol in 7mL of deionized water to form a transparent solution A; take 0.697g of cobalt acetate tetrahydrate solid and add it to the above transparent solution A while stirring, and mix well to obtain solution B; 9.08g of formaldehyde solution with a mass concentration of 37% was stirred for 5h to prepare a sol-gel precursor. Take 2.128ml of zirconium oxynitrate solution of 20mg / ml (based on the mass of metal zirconium), add 1.5M dilute ammonia solution drop by drop in a 60°C water bath and stir to adjust the pH of the zirconium oxynitrate / ammonia water complex to 7 . The prepared zirconium oxynitrate / ammonia water complex was added dropwise to the sol-gel precursor under vigorous stirring to obtain a homogeneous mixture C, and 3 mL of ammonia water with a mass concentration of 28% was added dropwise in an environment of 20 ° C and continued to seal and stir, and the reaction Gel D was formed; the gel D was transferred to a vacuum drying oven for...
Embodiment 3
[0053] Dissolve 6.16g of resorcinol in 7mL of deionized water to form a transparent solution A; take 0.697g of cobalt acetate tetrahydrate solid and add it to the above transparent solution A while stirring, and mix well to obtain solution B; 9.08g of formaldehyde solution with a mass concentration of 37% was stirred for 5h to prepare a sol-gel precursor. Take 2.128ml of zirconium oxychloride solution of 20mg / ml (based on the mass of metal zirconium), add 1.5M dilute ammonia solution drop by drop in a water bath at 60°C and stir to adjust the pH of the zirconium oxychloride / ammonia water complex =7. The prepared zirconium oxychloride / ammonia water complex was added dropwise to the sol-gel precursor under vigorous stirring to obtain a homogeneous mixture C, and 3 mL of ammonia water with a mass concentration of 28% was added dropwise in an environment of 20 ° C and continued to seal and stir. The reaction forms gel D; transfer the gel D to a vacuum drying oven for 7 days of va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com