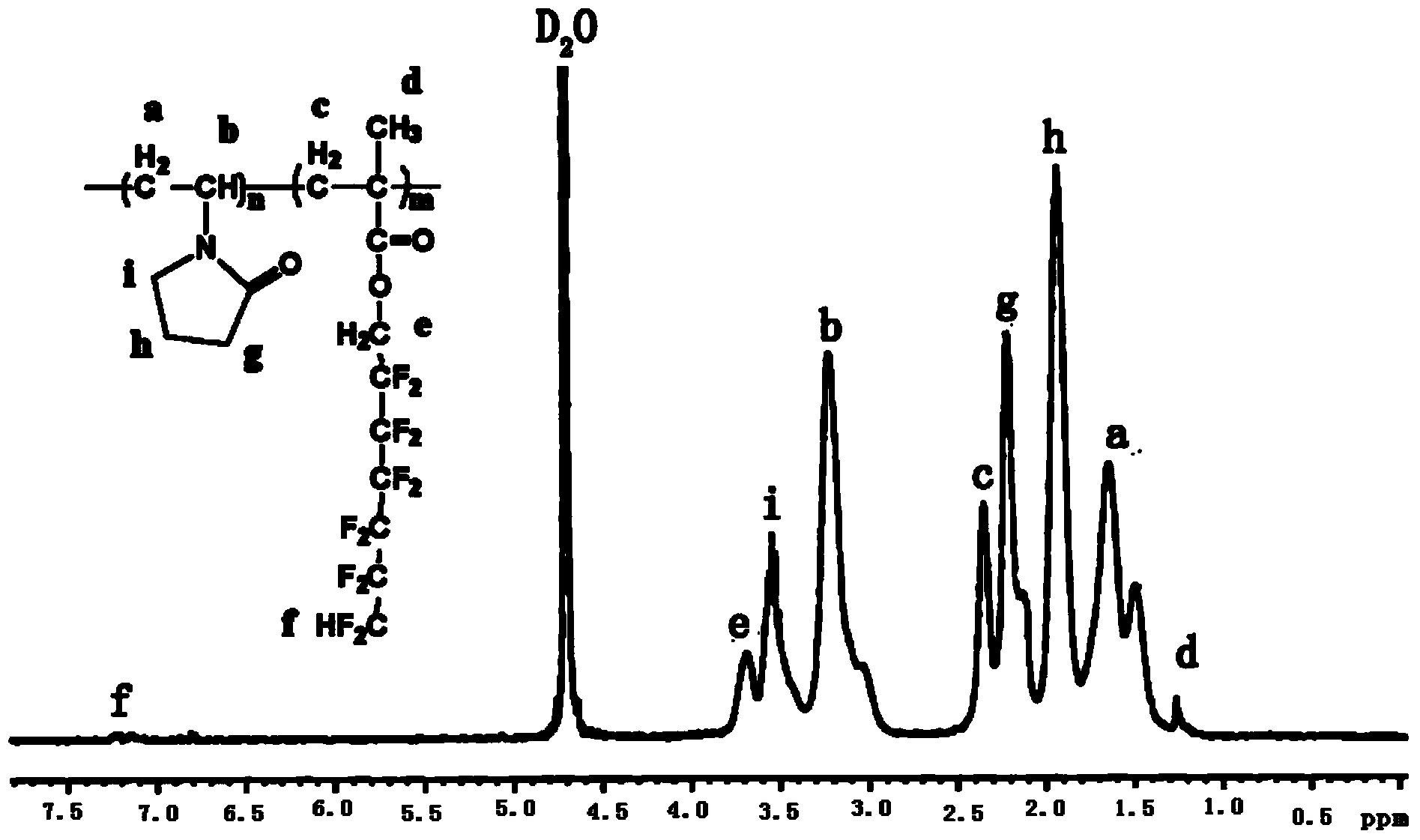
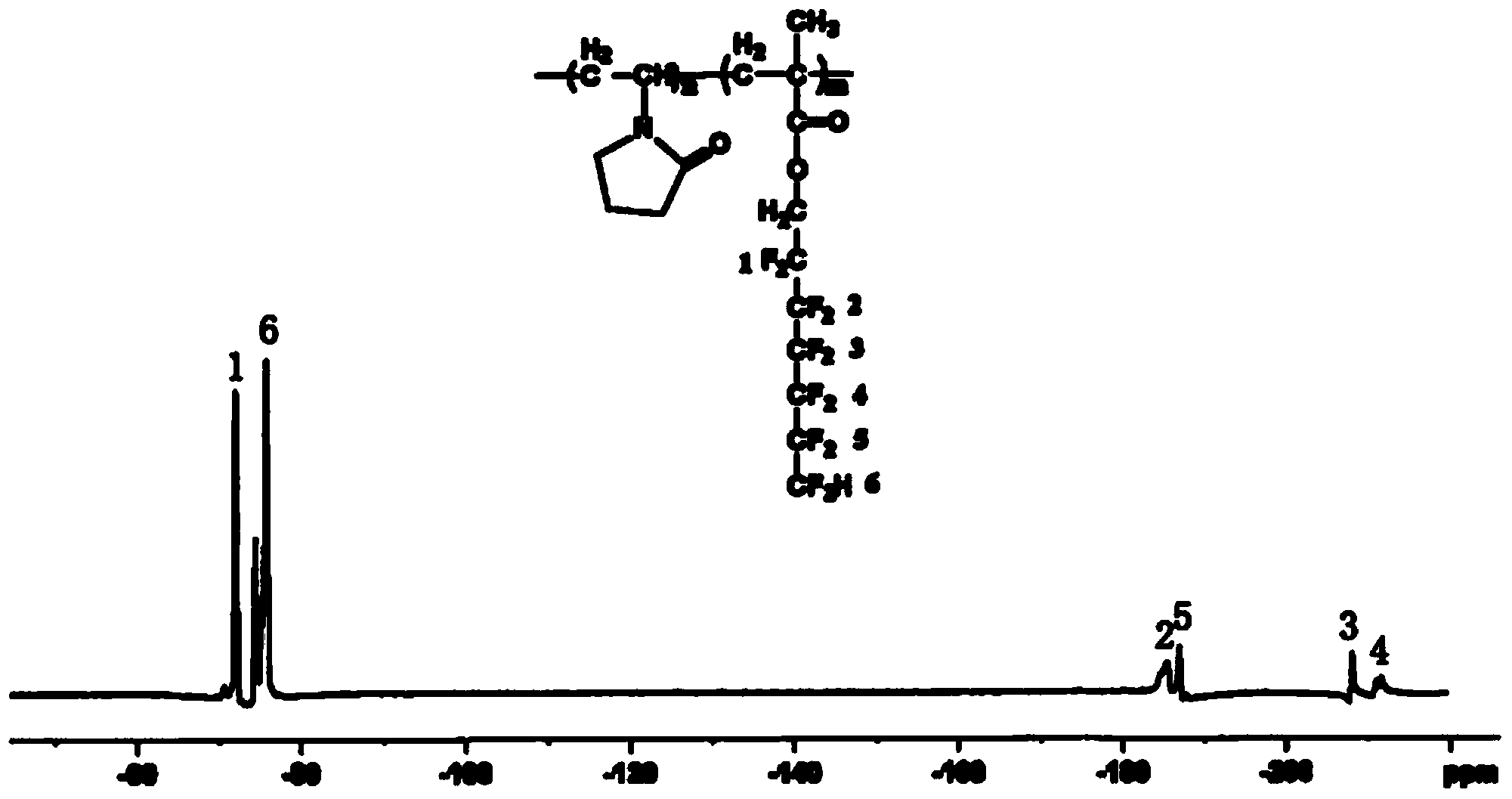
Water-soluble fluorine-containing modified N-NVP polymer and preparation method thereof
A technology of vinylpyrrolidone and water-soluble polymers, which is applied in the field of water-soluble polymer synthesis, can solve the problems of low product surface activity and high content of hydrocarbon side chain monomers, and achieve simple and easy operation of the product post-processing process, raw materials Easy to obtain, high conversion rate of monomer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A preparation method of a water-soluble polymer containing fluorine-modified N-vinylpyrrolidone, the steps are as follows:
[0052] (1) Weigh 8.3g (0.07477mol) of N-vinylpyrrolidone (NVP) monomer, and measure 50mL of toluene, add it into a 100mL three-necked flask equipped with a nitrogen tube, a condenser tube and a stirring bar, and stir until completely dissolved;
[0053] (2) Weigh 0.09 g (0.225 mmol) of dodecafluoroheptyl methacrylate (DFMA), add it into a three-neck flask, fill it with nitrogen gas at room temperature, and fully stir until dissolved;
[0054] (3) Weigh 0.0839 g of azobisisobutyronitrile, add it to a three-neck flask, put it in a constant temperature water bath at 25°C, and blow nitrogen gas for 0.5 h;
[0055] (4) Raise the reaction temperature to 85°C and react for 8 hours to obtain a water-soluble polymer containing fluorine-modified N-vinylpyrrolidone.
[0056] Subsequent treatment: the water-soluble polymer of fluorine-modified N-vinylpyrrolid...
Embodiment 2
[0060] As described in Example 1, the difference is that the amount of N-vinylpyrrolidone monomer in step (1) is 11.1 g;
[0061] The feeding amount of monomer dodecafluoroheptyl methacrylate (DFMA) in step (2) is 0.2 g.
[0062] 10.012 g of water-soluble polymer powder containing fluorine-modified N-vinylpyrrolidone was obtained, and the yield was ≥88%. Molecular weight M w =(2.291±0.609)×10 5 g·mol -1 .
[0063] The water-soluble polymer containing fluorine-modified N-vinylpyrrolidone prepared in this example has high surface activity at room temperature, the concentration of its aqueous solution is 10 mg / mL, and the surface tension of the aqueous solution can be reduced to 33 mN / m.
Embodiment 3
[0065] As described in Example 1, the difference is that the amount of N-vinylpyrrolidone monomer in step (1) is 7.87g;
[0066] The feeding amount of monomer dodecafluoroheptyl methacrylate (DFMA) in step (2) is 0.2 g.
[0067] 6.983 g of water-soluble polymer powder containing fluorine-modified N-vinylpyrrolidone was obtained, and the yield was ≥86%. Molecular weight M w =(2.380±0.426)×10 5 g·mol -1 .
[0068] The water-soluble polymer containing fluorine-modified N-vinylpyrrolidone prepared in this example has high surface activity at room temperature, the concentration of its aqueous solution is 10 mg / mL, and the surface tension of the aqueous solution can be reduced to 33 mN / m.
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com