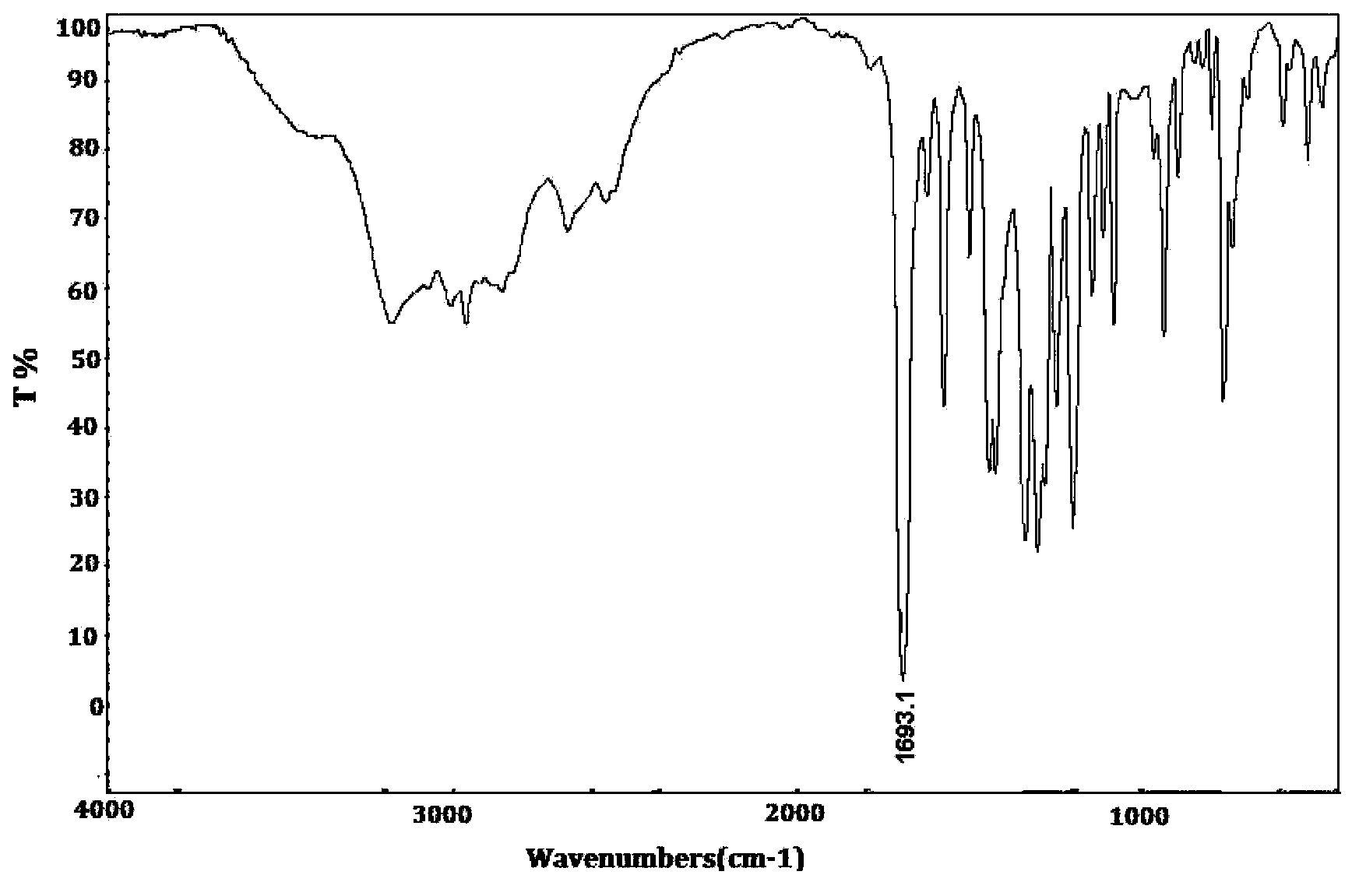
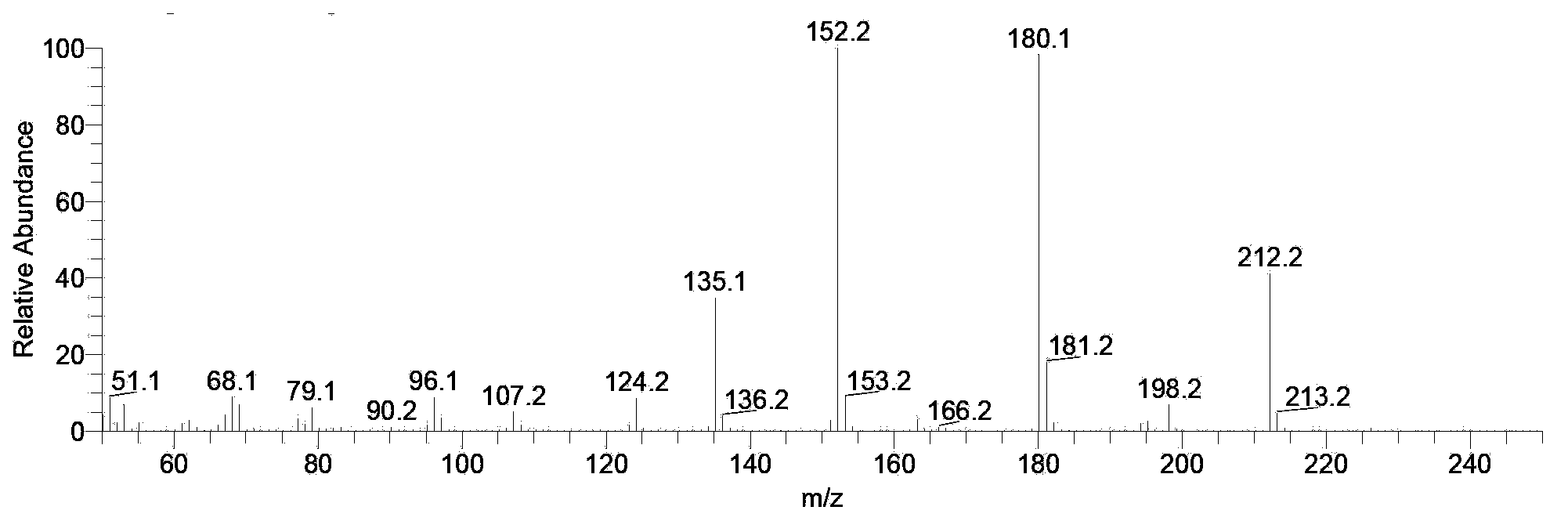
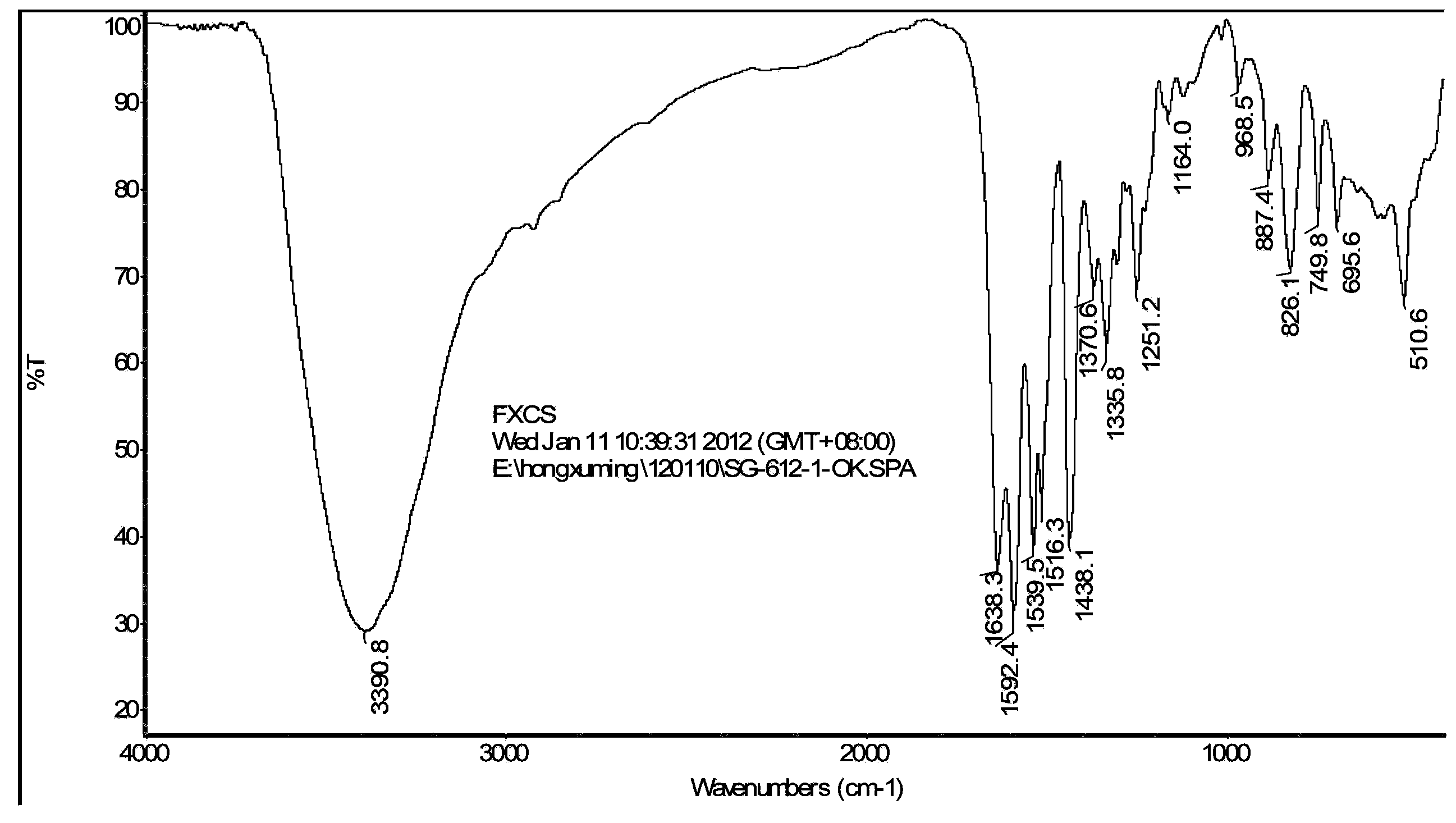
Monomers and intermediates of AB type hydroxyl modified high polymer and preparation methods of monomers and intermediates
A technology of hydroxyl and hydroxyl terephthalic acid, applied in the preparation of organic compounds, chemical instruments and methods, preparation of carboxylic acid esters, etc., can solve the problems such as weakening of the positive charge of the carbonyl carbonyl group of acyl chloride, difficulty in carrying out, and reduction in reaction ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1 Preparation of methyl 2-hydroxy-4-carboxybenzoate (α-MHT)
[0086] (1) Preparation of dimethyl hydroxy terephthalate (DMHT)
[0087] Add 15.00g HTA (0.082mol), 150mL methanol, 24.1mL SOCl to the reaction vessel 2 (0.330mol), heated, stirred and refluxed for 21 hours, then poured into a large amount of ice water, and a white precipitate was precipitated, adjusted to pH=8 with dilute ammonia water and filtered, and the filter cake was recrystallized with methanol to obtain 16.21g of white needle-shaped DMHT with a purity of 99.1%. Yield 94.16%.
[0088] (2) Preparation of α-MHT
[0089] Add 6.30g (0.030mol) DMHT and 5.00g NaOH (0.120mol) 200mL aqueous solution into the reaction vessel, stir in an ice-water bath, react at 0~5°C for 60min, pour into a beaker, adjust to pH = 8 with dilute hydrochloric acid, and filter out insoluble matter , add dilute hydrochloric acid to the filtrate to pH = 4.4, filter, dry the filter cake and recrystallize with methanol to ob...
Embodiment 2~7
[0090] The preparation of embodiment 2~7DMHT and α-MHT
[0091] (1) Preparation of dimethyl hydroxy terephthalate (DMHT)
[0092] Adopt the same operation of embodiment 1-(1) to synthesize DMHT, according to the parameter range described in table 1 (different amounts of SOCl 2 and different reaction times) to carry out experiments with different parameters, the results are shown in Table 1:
[0093] Table 1:
[0094] Example SOCl 2 equivalent Reflow time / h product purity / % Yield / % 2 2.99 7.5 DMHT 96.3 83.5 3 3.50 15 DMHT 98.6 89.4 Comparative example 1 1.5 0.5 β-Monoester 94.7 69.3
[0095] (2) Preparation of α-MHT
[0096] Using the same operation as in Example 1-(2) to synthesize α-MHT, experiment with different parameters according to the parameter range described in Table 2 (temperature, alkali dosage, reaction time, hydrolysis solvent), and the results are shown in Table 2:
[0097] Table 2:
[0098] ...
Embodiment 82
[0099] Example 82, Preparation of methyl 6-dihydroxy-4-carboxybenzoate (α-MDHT)
[0100] (1) Preparation of dimethyl 2,6-dihydroxyterephthalate (2,6-DMDHT)
[0101] Add 14.85g 2,6-DHTA (0.075mol), 150mL methanol, 22mL SOCl to the reaction vessel 2 (0.3mol), heated, stirred and refluxed for 21 hours, cooled and poured into a large amount of ice water, precipitated a white precipitate, adjusted to pH=8 with dilute ammonia water and filtered, and the filter cake was recrystallized with methanol to obtain 15.29g of 2,6-DMDHT, with a purity of 98.7% , yield 90.23%.
[0102] (2) Preparation of α-MDHT
[0103] Add 6.78g (0.030mol) 2,6-DMDHT and 5.00g NaOH (0.120mol) 200mL aqueous solution into the reaction vessel, stir in an ice-water bath, react at 2~5°C for 120min, then pour into a beaker, adjust the pH to 8 with dilute hydrochloric acid, Filter out insoluble matter, add dilute hydrochloric acid to the filtrate to pH = 4.4, filter, dry the filter cake and recrystallize with meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com