Multivalence pneumococcus capsular polysaccharide-protein conjugate composition and preparation method thereof
A protein conjugate and capsular polysaccharide technology, applied in the field of multivalent pneumococcal capsular polysaccharide-protein conjugate composition and its preparation, can solve the problems of limited protection range and lack of protection of serotypes, and reduce vaccination The number of stitches, the convenience of industrialized mass production, and the effect of covering a wide range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of pneumococcal capsular polysaccharide stock solution
[0030] 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F serotype pneumonia All strains of Streptococcus pneumoniae were obtained from the China Institute for the Control of Pharmaceutical and Biological Products. The above strains were subcultured to establish the original seed bank, main seed bank and working seed bank. The generations were as follows: the original seed batch was the first generation, the main seed batch was the fourth generation, and the working seed batch was the eighth generation. From the opening of the working seed batch to the cultivation in the inoculated fermenter, the passage should not exceed 5 generations. The seeds of each generation use milk powder as a freeze-drying protective agent and freeze-dried for preservation. Take the working seeds and pick the strains by streaking on the soybean peptone solid med...
Embodiment 2
[0035] Example 2 Preparation of polyvalent pneumococcal capsular polysaccharide-protein conjugate composition
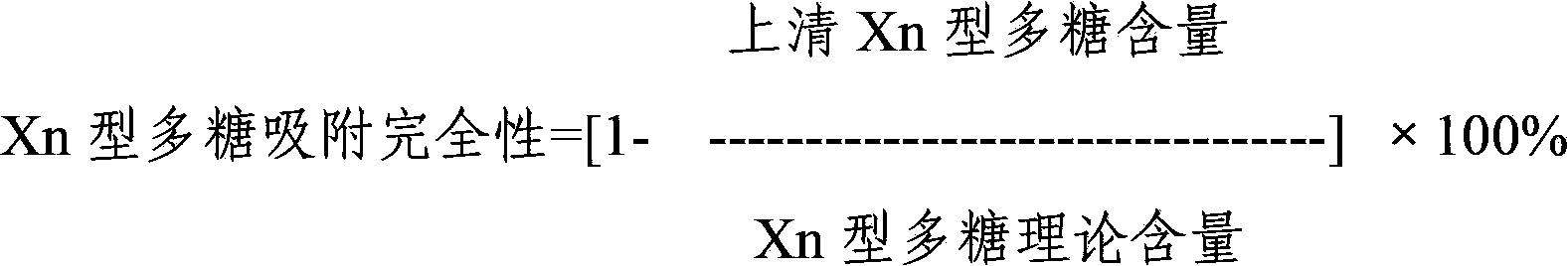
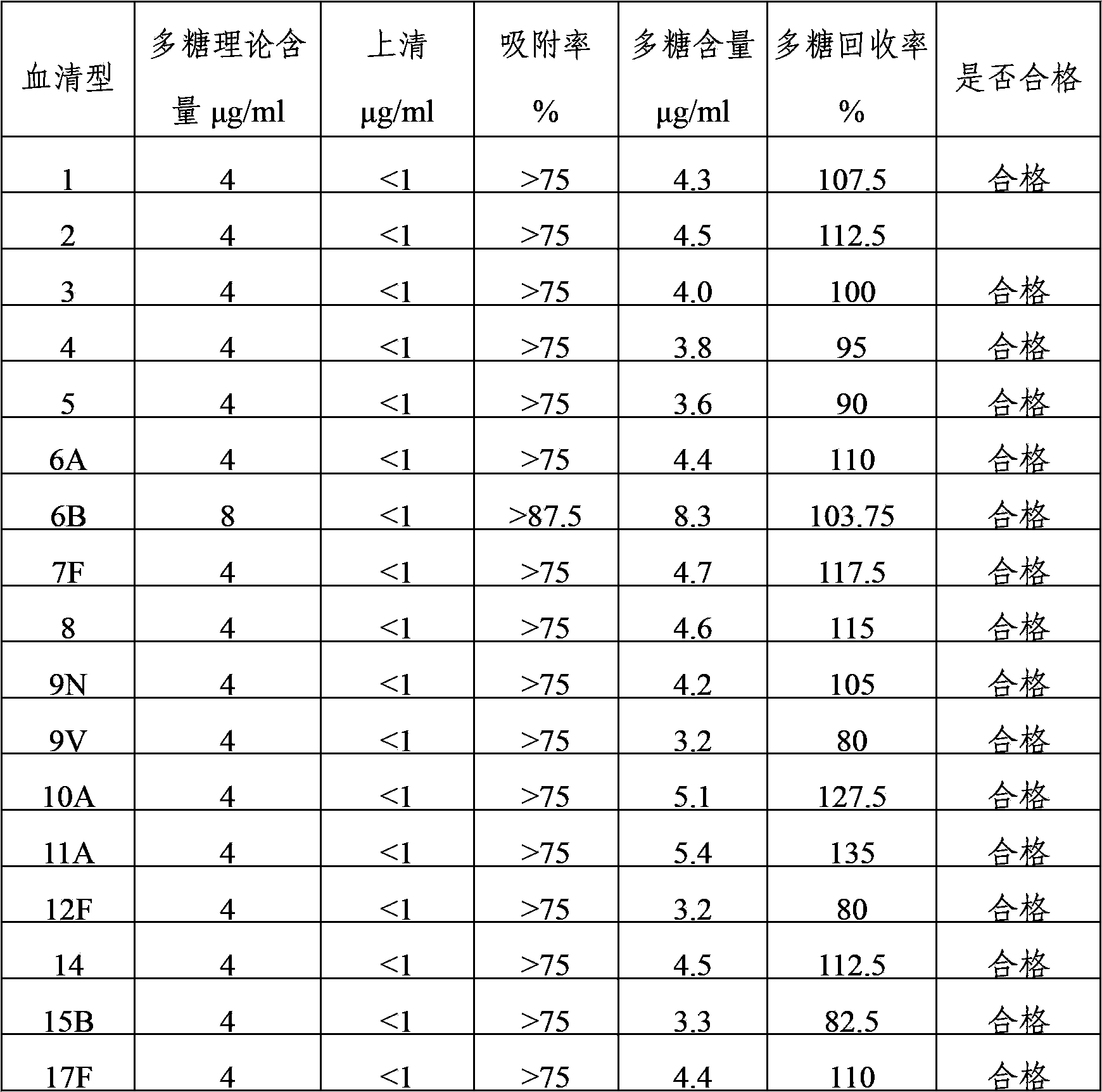
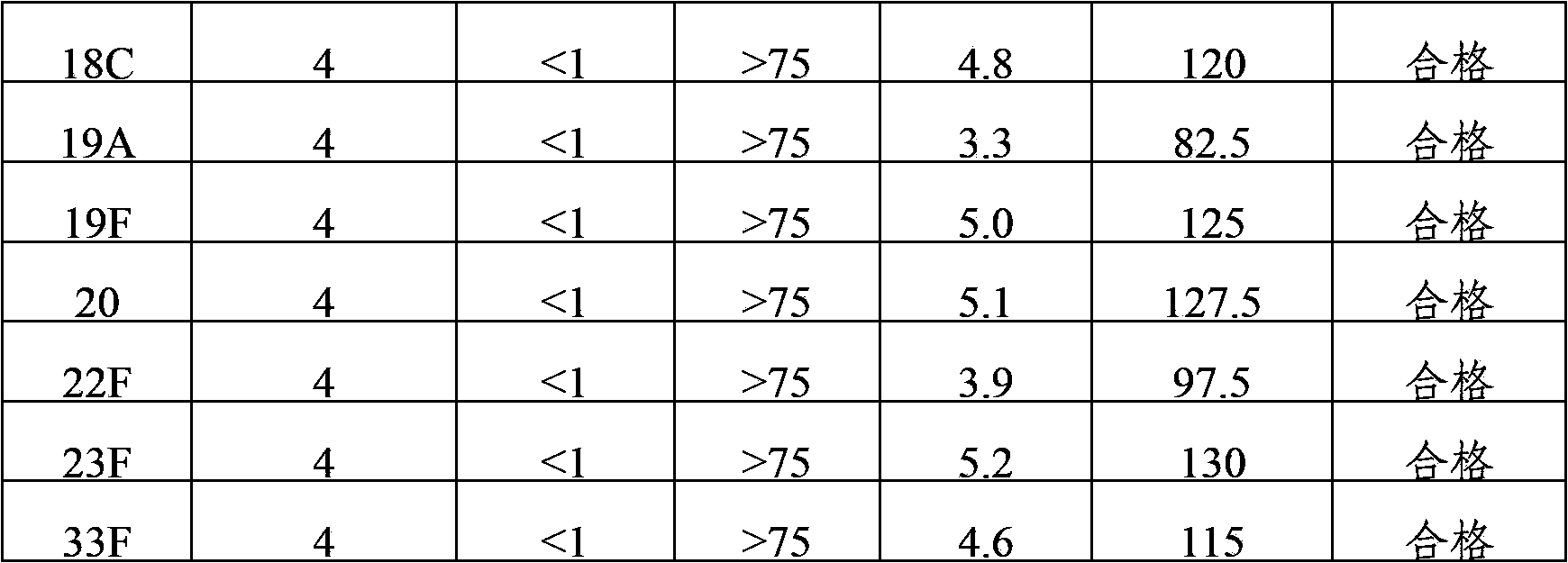
[0036] The 24 kinds of pneumococcal capsular polysaccharide stock solutions prepared in Example 1 were acidified and degraded with hydrochloric acid, and then sodium periodate was added to the polysaccharide for oxidation. After oxidation, the activation degree of the polysaccharide was detected, and then polysaccharide: protein = 0.4-3 Add the purified diphtheria toxoid CRM197 protein at a mass ratio of :1 for binding, and then add sodium borohydride to terminate the binding reaction to prepare pneumococcal capsular polysaccharide conjugates. The conjugates are dialyzed by ultrafiltration to remove unbound proteins and polysaccharide, after purification, use a 0.22 μm filter to sterilize and filter, which is the monovalent conjugate of pneumococcal capsular polysaccharide-protein.
[0037] 1, 2, 3, 4, 5, 6A, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F,...
Embodiment 3
[0038] Example 3 Preparation of multivalent pneumococcal capsular polysaccharide conjugate vaccine
[0039] 1, 2, 3, 4, 5, 6A, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and The pneumococcal capsular polysaccharide-protein monovalent conjugate of 33F serotype is mixed according to the final polysaccharide concentration of 4 μg / ml, and the 6B conjugate is mixed according to the final polysaccharide concentration of 8 μg / ml, and aluminum phosphate adjuvant is added to make 24-valent pneumococcal capsule Polysaccharide conjugate vaccine with an aluminum content of 0.4 mg / ml.
[0040] The 24-valent pneumococcal capsular polysaccharide conjugate vaccine prepared above was made into a freeze-dried dosage form by low-temperature drying, and reconstituted with physiological saline before use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com