Moxifloxacin hydrochloride tablet and preparation method thereof
A technology of moxifloxacin hydrochloride and tablet, which is applied in the field of moxifloxacin hydrochloride tablet and its preparation, can solve the problems affecting the disintegration time limit of the tablet, and achieve the advantages of convenient industrialization and clinical use, low price and beautiful appearance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
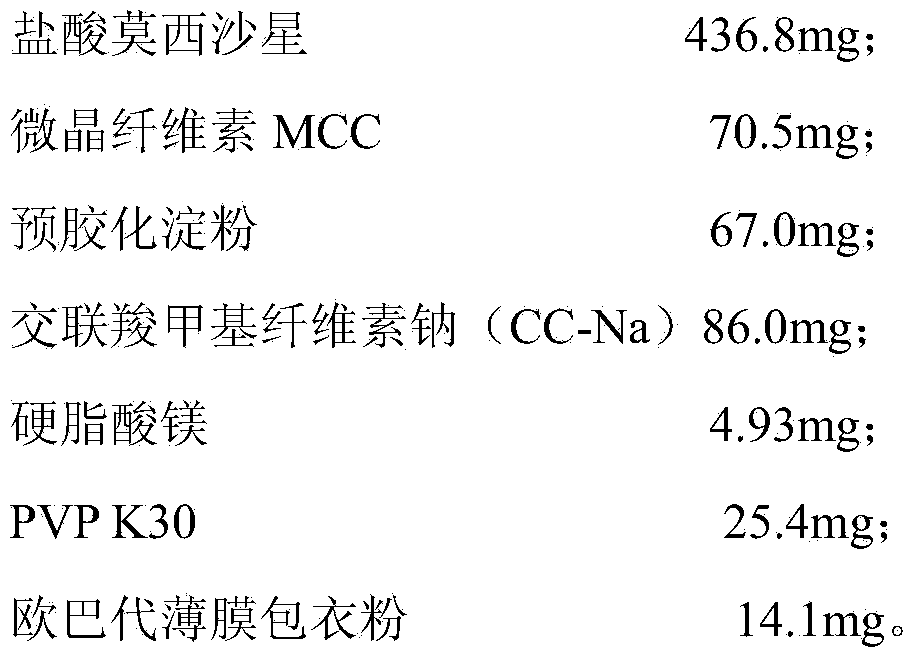
[0030]
[0031] The preparation method of moxifloxacin hydrochloride tablets in this embodiment is as follows:
[0032] 1. Pass moxifloxacin hydrochloride through an 80 mesh sieve and set aside;
[0033] 2. Accurately weigh moxifloxacin hydrochloride, MCC, pregelatinized starch and one-third of the prescription amount of CC-Na according to the prescription amount and mix them through a 120-mesh sieve, and then mix well;
[0034] 3. The concentration of PVP K30 prepared by adding water is 8.0%wt, and the amount of 100 pieces of 28ml binder is passed through a 16-mesh sieve, and dried at 50℃ for 1.5 hours;
[0035] 4. Add the remaining CC-Na and magnesium stearate, mix well, press the tablet after passing the test of the intermediate, and coat it with Opadry film coating powder.
Embodiment 2
[0037] In this example, the moxifloxacin hydrochloride tablets, in terms of weight fraction, have the following raw materials:
[0038]
[0039]
[0040] The preparation method of moxifloxacin hydrochloride tablets in this embodiment is as follows:
[0041] 1. Pass moxifloxacin hydrochloride through an 80 mesh sieve and set aside;
[0042] 2. Accurately weigh moxifloxacin hydrochloride, MCC, pregelatinized starch, and half of the prescription amount of CC-Na according to the prescription amount, mix them with a 200-mesh sieve, and then mix;
[0043] 3. The concentration of PVP K30 prepared by adding water is 6.0%wt, and 100 pieces of 39ml binder are used to pass through a 16-mesh sieve to granulate, and dry at 60°C for 1.0 hour;
[0044] 4. Add the remaining CC-Na, and magnesium stearate, mix well, press the tablet after passing the intermediate test, and then coat it with Opadry film coating powder.
Embodiment 3
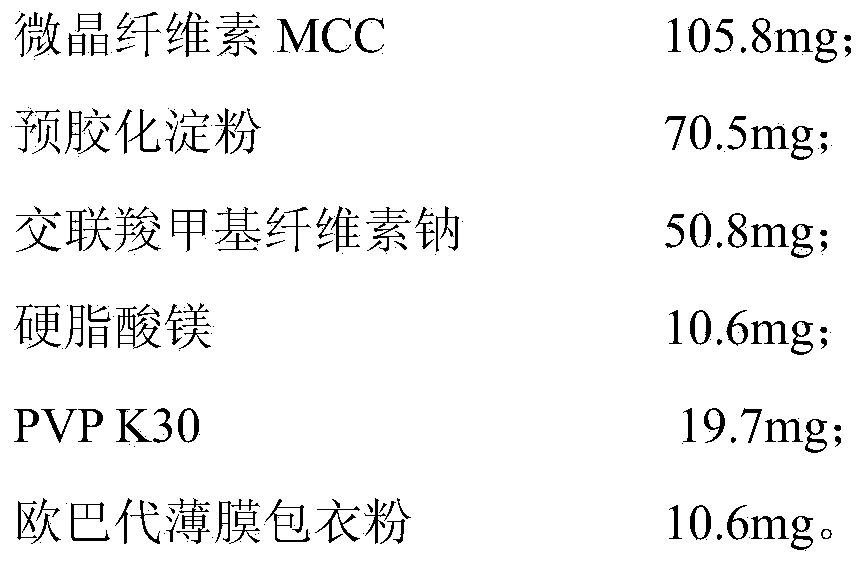
[0046] In this example, the moxifloxacin hydrochloride tablets, in terms of weight fraction, consist of the following raw materials:
[0047]
[0048] The preparation method of moxifloxacin hydrochloride tablets in this embodiment is as follows:
[0049] 1. Pass moxifloxacin hydrochloride through an 80 mesh sieve and set aside;
[0050] 2. Accurately weigh moxifloxacin hydrochloride, MCC, pregelatinized starch, and a quarter of the prescription amount of CC-Na according to the prescription amount and mix them through a 100-mesh sieve, and then mix;
[0051] 3. The concentration of PVP K30 prepared by adding water is 10.0%wt, and 100 pieces of 23ml binder are used to pass through a 16-mesh sieve to granulate, and then dried at 40℃ for 2 hours;
[0052] 4. Add the remaining CC-Na and magnesium stearate, mix well, press the tablet after passing the intermediate test, and coat with Opadry film coating powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com