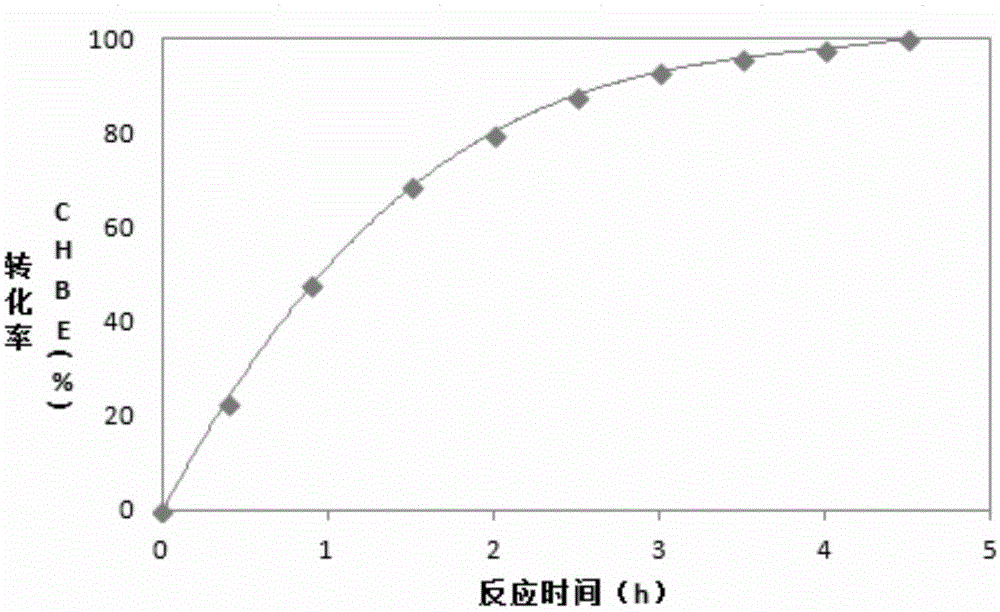
Alcohol dehydrogenase mutant and its application
A technology of alcohol dehydrogenase and mutants, applied in the direction of oxidoreductase, introduction of foreign genetic material by using vectors, recombinant DNA technology, etc., can solve the problems of enzyme activity loss and achieve high conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Construction of Alcohol Dehydrogenase Gene.
[0040] 1. Acquisition of alcohol dehydrogenase gene:
[0041] Candida magnoliae (Candida magnoliaeATCC12573), medium YPD (g L -1 ): Yeast extract 10g, peptone 20g, glucose 20g, add distilled water to 1L.
[0042] Candida magnoliae (Candida magnoliae ATCC12573) was inoculated in 5 mL of LYPD liquid medium and cultured at 30°C until the logarithmic growth phase, and the genome was extracted using a genomic DNA extraction kit (Beijing Tianwei Bioengineering Co., Ltd. Yeast Genome Extraction Kit, GD2415YeastgDNAKit).
[0043] The primers used to construct the expression vectors and the primers with restriction sites are as follows:
[0044] The upstream primer is (NdeIsiteisunderlined):
[0045] 5'-GGAATTC CATATG ACGACTACTTCAAATGCGCTCGTCAC-3'
[0046] The downstream primer is (EcoRIsiteisunderlined):
[0047] 5'-CCG GAATTC CTAAGCAATCAAGCCATTGTCGACCAC-3'
[0048] All primers were synthesized by Shanghai Meiji...
Embodiment 2
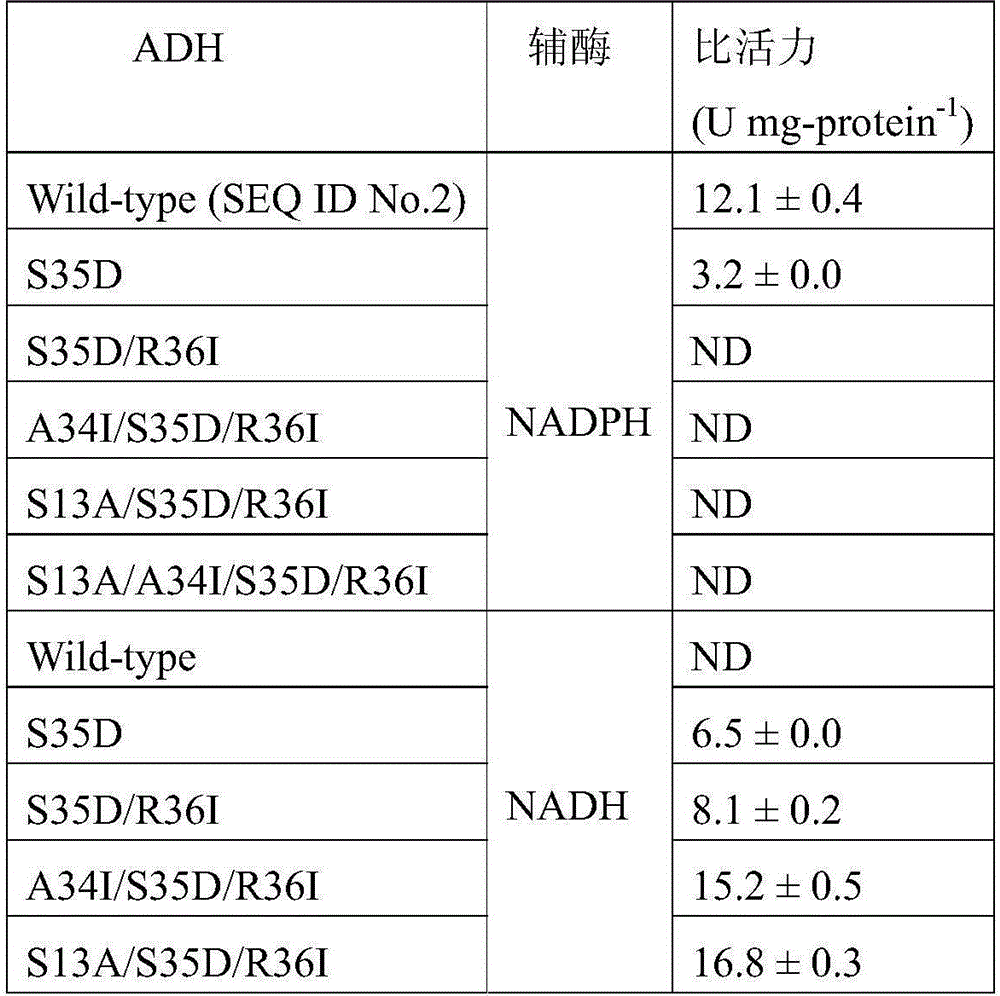
[0054] Example 2: Construction of Alcohol Dehydrogenase Mutant Gene.
[0055] 1. Site-directed mutation
[0056] The amino acid residues located at Ser13, Ala34, Ser35, and Arg36 positions were subjected to site-directed mutagenesis using Stratagene's rapid conversion site-directed mutagenesis kit. The primers are designed as follows (both are described in the 5'-3' direction, and the underline represents the mutation site):
[0057] Ser35Asp (pET24a-ADH recombinant plasmid as template)
[0058] S35D-1:CAGTGTTACGCTGGCC GAC CGCAGTGTTG
[0059] S35D-2:CAACACTGCG GTC GGCCAGCGTAACACTG
[0060] Ser35Asp / Arg36Ile (mutant Ser35Asp as template)
[0061] S35D / R36I-1:CAGTGTTACGCTGGCCGAC ATC AGTGTTG
[0062] S35D / R36I-2:CAACACT GAT GTCGGCCAGCGTAACACTG
[0063] Ala34Ile / Ser35Asp / Arg36Ile (mutant Ser35Asp / Arg36Ile as template)
[0064] A34I / S35D / R36I-1:CAGTGTTACGCTG ATC GACCGCAGTGTTG
[0065] A34I / S35D / R36I-2:CAACACTGCGGTC GAT CAGCGTAACACTG
[0066] Ser13Ala / Ser35Asp / A...
Embodiment 3
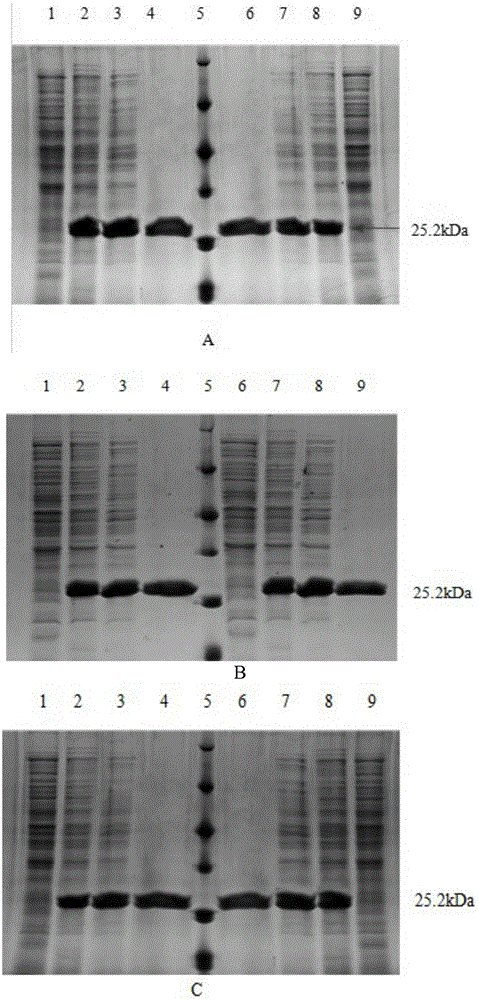
[0080] Example 3: Purification of alcohol dehydrogenase and mutant enzymes thereof.
[0081] 1. Preparation of crude enzyme solution: take the induced LB culture medium at 8000r·min-1, centrifuge for 15min to collect the bacteria, wash twice with sterile water, resuspend the bacteria in pH6.220mmol·L-1 phosphate buffer Cells were disrupted ultrasonically in an ice bath. Centrifuge the ultrasonically crushed sample at 12,000r·min-1 at 4°C for 10min to obtain the supernatant, which is the crude enzyme solution.
[0082] 2. Ammonium sulfate precipitation: put the crude enzyme solution in an ice bath, slowly add saturated ammonium sulfate solution dropwise under magnetic stirring until the final concentration of ammonium sulfate is 30%, and stir overnight at 4°C. Centrifuge and take the supernatant. Also under the condition of ice bath, saturated ammonium sulfate solution was slowly added dropwise to the supernatant to a final concentration of 60%, and stirred overnight at 4°C. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com