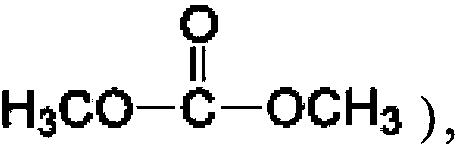
Method for simultaneously producing dimethyl carbonate and dimethyl ether through urea alcoholysis process, catalyst used thereby, and preparation method of catalyst
A technology of dimethyl carbonate and catalyst, which is applied in the field of urea alcoholysis for co-production of dimethyl carbonate and dimethyl ether, which can solve the problems of complex reactor structure and operating conditions, low conversion rate of raw materials and low yield of dimethyl carbonate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
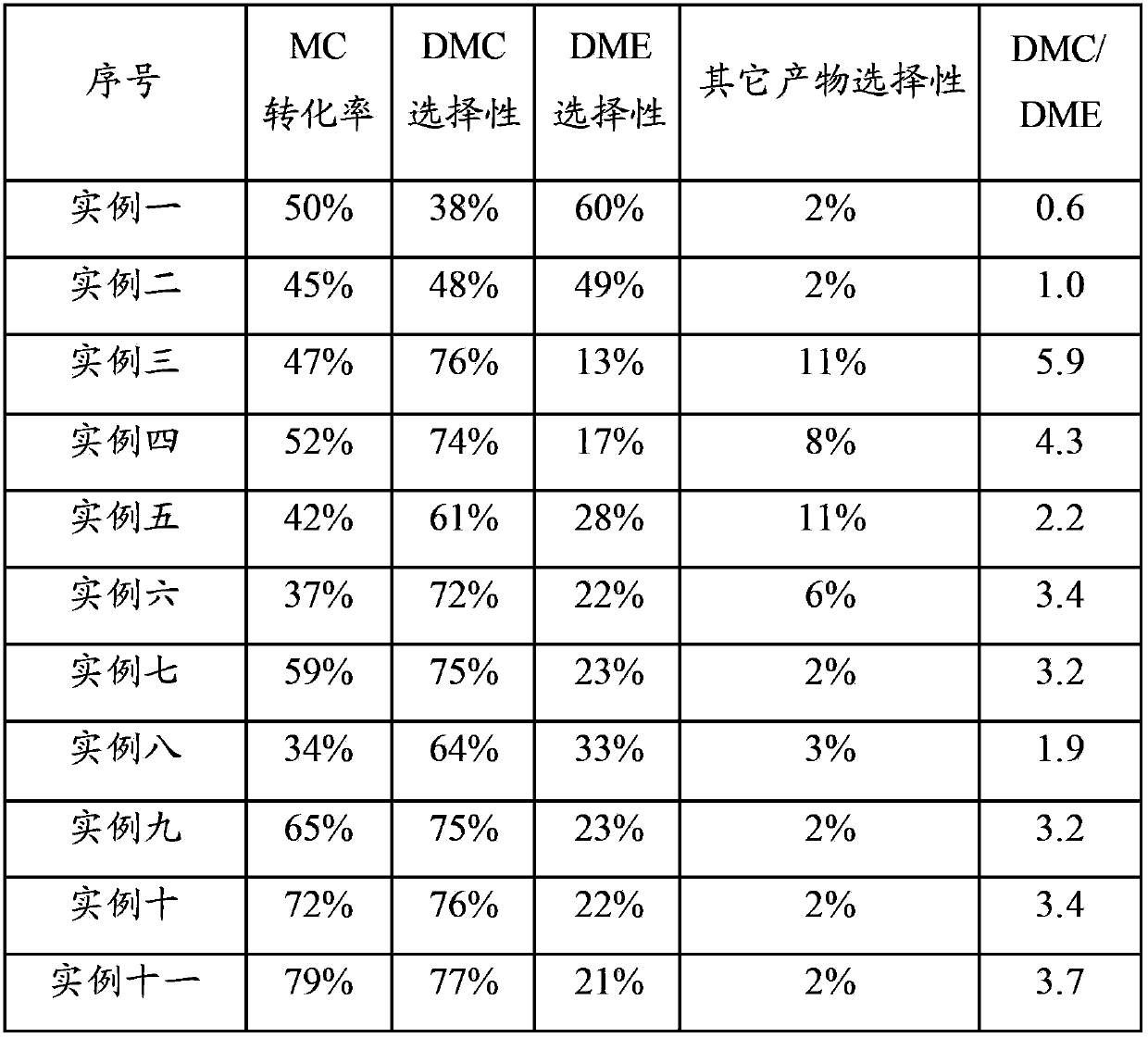
Examples
Embodiment 1
[0101] Raw materials: Mg(NO 3 ) 2 ·6H 2 O, Al 2 o 3 ,Deionized water.
[0102] Preparation process: 13.3gMg(NO 3 ) 2 ·6H 2 O was dissolved in 30ml of water to form a solution, and 7.9g of dried Al 2 o 3 Sample, mix the two evenly and let stand for 60 minutes. Stir and dry under an infrared lamp, and then place in an oven at 110°C to dry. Calcined in air atmosphere at 850°C for 8 hours.
[0103] Product: The obtained catalyst sample contains about 20wt% MgO, Al 2 o 3 About 80% by weight.
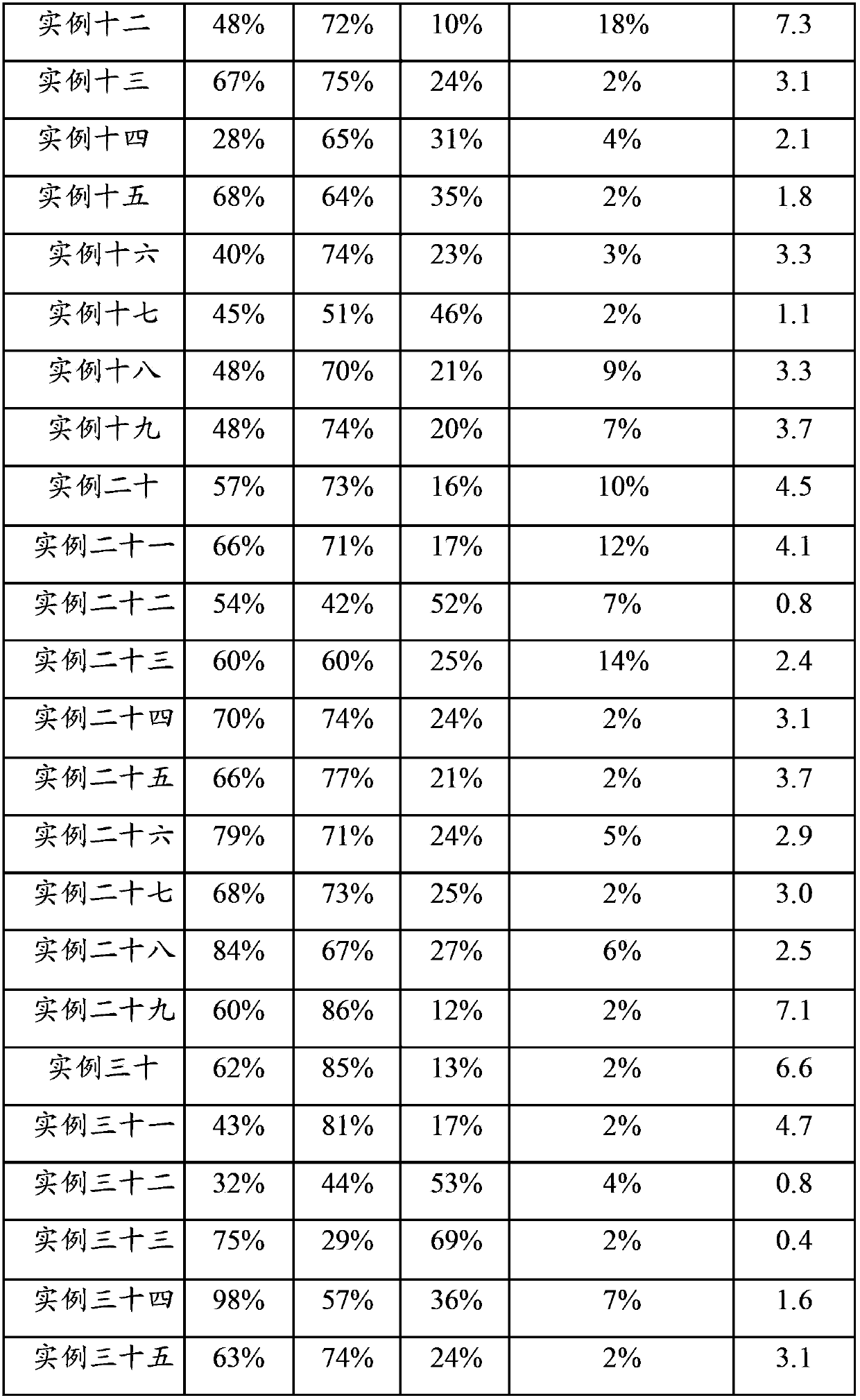
example 2
[0105]Raw material: Ni(NO 3 ) 2 ·6H 2 O, Al 2 o 3 ,Deionized water.
[0106] Preparation process: 25.3g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 38ml water to form a solution, and 11.5g of dried Al 2 o 3 Sample, mix the two evenly and let stand for 30 minutes. Stir and dry under an infrared lamp, and then place in an oven at 100°C to dry. Calcined in air atmosphere at 800°C for 6 hours.
[0107] Product: The obtained catalyst sample contains about 36wt% NiO, Al 2 o 3 About 64% by weight.
example 3
[0109] Raw material: LiNO 3 , Zn(NO 3 ) 2 ·6H 2 O, Al 2 o 3 ,Deionized water.
[0110] Preparation process: 12.5g Zn(NO 3 ) 2 ·6H 2 O was dissolved in 20ml of water to form a solution, and 5.6g of dried Al 2 o 3 Sample, mix the two evenly and let stand for 60 minutes. Stir and dry under an infrared lamp, and then place in an oven at 100°C to dry. Sample A was obtained by calcining at 850° C. in an air atmosphere for 12 hours. 0.8g LiNO 3 Dissolve it in 19ml of water to make a solution, mix it evenly with sample A, dry it under an infrared lamp, and then place it in an oven at 120°C. Finally, it was calcined in an air atmosphere at 500° C. for 3 hours.
[0111] Product: The resulting catalyst sample contains Li 2 O about 4wt%, ZnO about 37wt%, Al 2 o 3 About 59% by weight.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com