Preparation method of DCs, and application of DCs in preparing anti-tumor cell preparation
A cell and nuclear cell technology, applied in the application field of mature DC cells in the preparation of anti-tumor cell preparations, can solve the problems of failing to achieve clinical efficacy, inhibiting the secretion of IL-12 by DC cells, etc., achieving strong cell migration ability and increasing the number of cells , high maturity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of adherent mononuclear cells
[0047] (1) Add the coating solution containing 1-10 μg / mL CD14 monoclonal antibody to the culture bottle dish made of polystyrene, polyvinyl chloride, polyethylene, etc., and wrap the culture bottle dish with tin foil. Store in the dark, lay flat, and incubate overnight at 4°C; wherein, the type of CD14 monoclonal antibody used can be any one of IgG1, IgG2a, IgG2b, IgG3, IgM and IgA;
[0048] (2) On the first day, draw 50-100 mL of peripheral blood from normal people or tumor patients under sterile conditions, or take 80-100 mL of peripheral blood mononuclear cells from normal people or tumor patients preliminarily separated by blood collection (ie, apheresis). into a 50mL centrifuge tube, add an equal volume of normal saline to dilute to obtain a diluted sample; then transfer the diluted sample to a 2:1 or 1:1 volume ratio of the diluted sample to the lymphocyte separation solution. Above the lymphocyte separation...
Embodiment 2
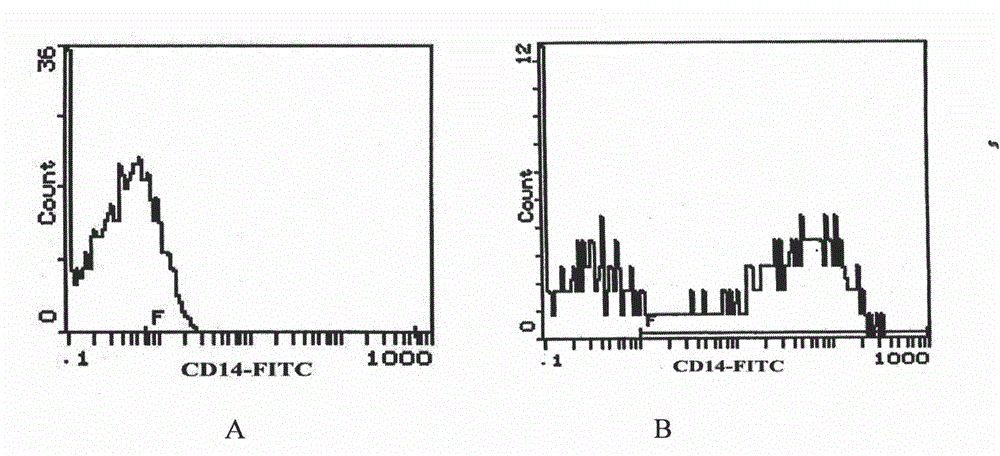
[0053] Example 2: The CD14 monoclonal antibody coating method can increase the adherence ability of mononuclear cells and increase the number of adherent cells, thereby helping to reduce the influence of impurity cells on their transformation into immature DCs
[0054] (1) Prepare mononuclear cells according to the method of Example 1, grouping:
[0055] Group 1: Press the mononuclear cells into 2~4×10 6 / mL was planted in a culture bottle dish made of polystyrene, polyvinyl chloride or polyethylene and other materials coated with CD14 monoclonal antibody;
[0056] Group 2: Press the mononuclear cells into 2~4×10 6 / mL planted in petri dishes made of polystyrene, polyvinyl chloride or polyethylene;
[0057] After 2 to 4 hours, the supernatant was discarded, and the wall of the bottle was washed with normal saline, D-Hanks solution or PBS, and the adherent mononuclear cells were obtained;
[0058] (2) Comparison of CD14 obtained by the two methods + Number and purity of mon...
Embodiment 3
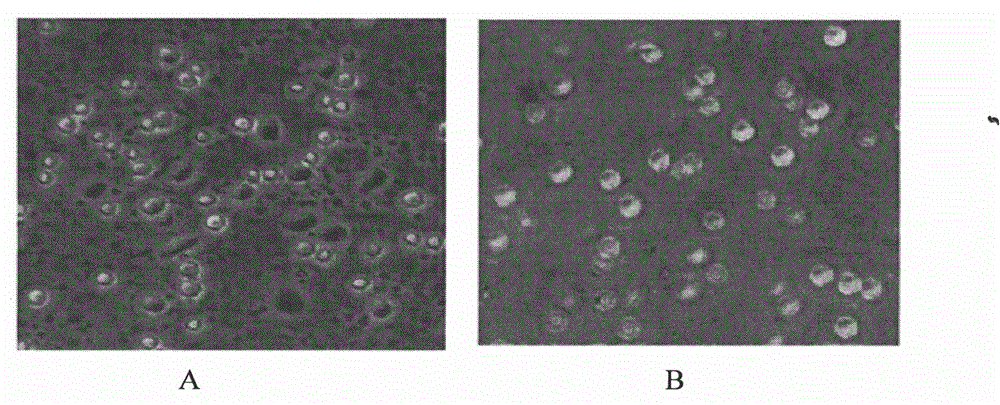
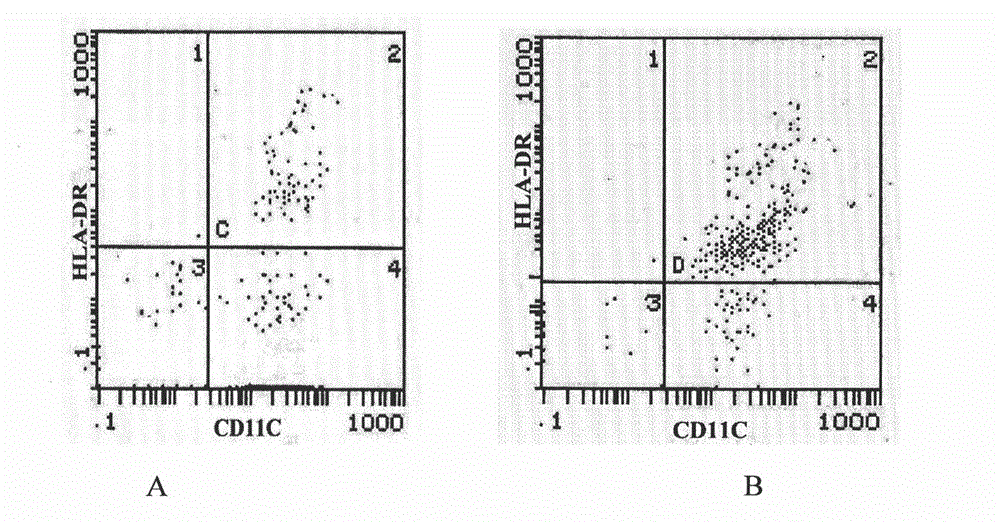
[0061] Example 3: Culture of Adherent Cells Using a Combination of GM-CSF, IL-4 and IFN-α Cytokines
[0062] (1) After adhering the mononuclear cells prepared by the method of Example 1 for 2 to 4 hours, aspirate and discard the supernatant, and gently rinse the wall of the bottle with normal saline, D-Hanks solution or PBS for 3 times, and aspirate and discard the supernatant. Add the original volume of medium GT-T551 / AIM V / X-VIVO, 500-1000U / mL GM-CSF, 500-1000U / mL IL-4, 500-1000U / mL IFN-α and 1-5% Autologous plasma or AB plasma, in 5% CO 2 , incubate at 37°C;
[0063] (2) On the third day, add double volume of medium GT-T551 / AIM V / X-VIVO, 500-1000U / mL GM-CSF, 500-1000U / mL IL-4, 500-1000U / mL IFN- Alpha and 1 to 5% autologous plasma or AB plasma in 5% CO 2 , incubate at 37°C;
[0064] (3) On the 5th day, the adherent cells were differentiated into immature DC cells; the immature DC cells grown in suspension were collected and washed by centrifugation at 600×g to obtain imm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com