Reduced glutathione fluorescent probe with pyrazoline as maternal body
A glutathione and fluorescent probe technology, applied in the field of fluorescent probes, can solve the problem of lack of specificity of reduced glutathione, and achieve the effects of improving high selectivity, high sensitivity, and enhancing membrane permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of 2–(1,5-diphenyl-4,5-dihydro-1H-pyrazole-3-one from 1-(2-hydroxyphenyl)-3-phenylprop-2-en-1-one Base) phenyl acrylate reacts with reduced glutathione to generate the reaction process of pyrazoline glutathione derivative as shown in the following formula:
[0023]
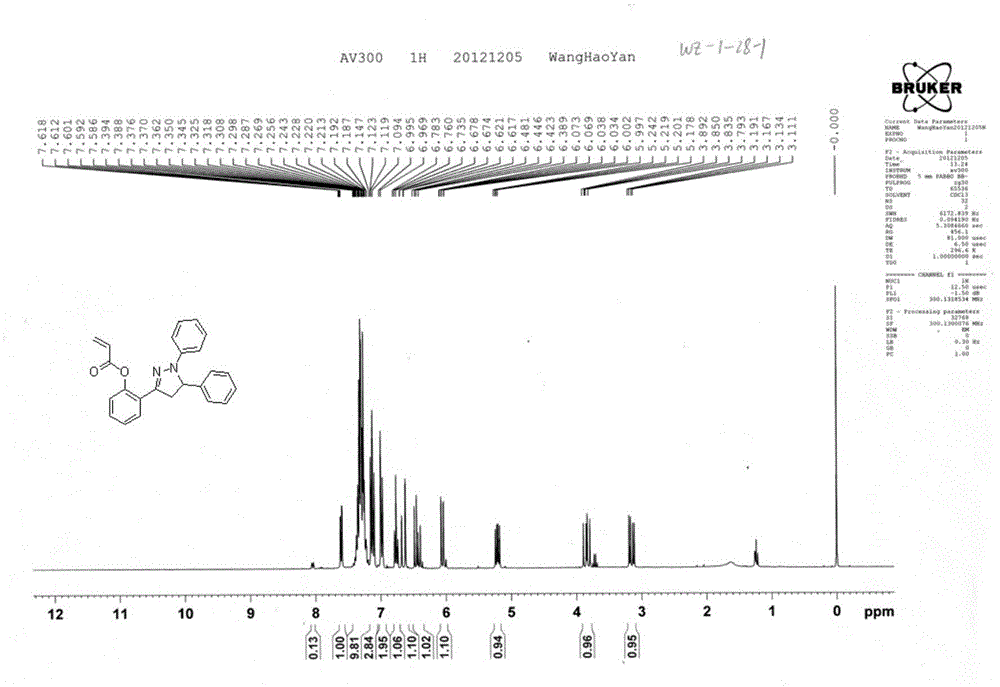
[0024] Add chalcone (0.224g, 1.0mmol) (1), 0.162g phenylhydrazine (2) (1.5mmol), sodium hydroxide (0.12g, 3.0mmol) to 15mL of absolute ethanol, heat to reflux, continue React for about four hours, cool down after the reaction is complete, add 100mL of water to the residue, adjust the pH value to 5, continue to extract the water phase with dichloromethane three times, combine the organic phase, dry with anhydrous magnesium sulfate, petroleum ether: dichloromethane = 5:1 is the eluent, the light yellow solid (3) 0.144g in column chromatography, melting point: 167-168°C, yield: 45.8%.
[0025] H NMR spectrum determination: 1 H NMR (300MHz, CDCl 3 ):δ3.25(dd,1H,J=7.5,17.1Hz,4-H trans ),3.95(dd,...
Embodiment 2
[0034] Add 10 mL of prepared 2–(1,5-diphenyl-4,5-dihydro-1H-pyrazol-3-yl)phenylacrylate derivative to PBS / ethanol buffer solution with a micro-syringe Add 10 equivalents of Cys, Hcy, GSH, Arg, Asp, Glu, Gly, His, Lys, Ser, Thr, Trp, Tyr, Val, KNO to the solution of (20mM PBS, pH=7.4, 3:7, volume ratio) 3 , Ca(NO 3 ) 2 ,NaNO 3 ,Mg(NO 3 ) 2 , Zn(NO 3 ) 2 ,Fe(NO 3 ) 3 , the aqueous solution of hydrogen peroxide and glucose, after 12 hours of action, the fluorescence spectrophotometry test shows that 2–(1,5-diphenyl-4,5-dihydro-1H-pyrazol-3-yl)phenyl Acrylate derivatives have good selectivity to reduced glutathione, and the control before and after adding reduced glutathione shows a strong fluorescence enhancement effect.
[0035] see results figure 1 .
Embodiment 3
[0037] Intracellular fluorescence imaging test:
[0038] The experiment was divided into two groups, control group A and B. Control group A: HeLa cells were treated with 3.0 μM 2–(1,5-diphenyl-4,5-dihydro-1H-pyrazole at 37°C -3-yl) in the cell culture medium of phenyl acrylate derivatives and cultivated for 0.5 hours; Control group B: HeLa cells were soaked in cell culture medium containing 100mM N-ethylmaleimide for 8 hours, washed with PBS buffer solution After three times, culture in the cell culture medium added with 3.0μM 2-(1,5-diphenyl-4,5-dihydro-1H-pyrazol-3-yl)phenylacrylate derivative for 0.5 hours .
[0039] Fluorescence imaging showed that 2–(1,5-diphenyl-4,5-dihydro-1H-pyrazol-3-yl)phenylacrylate derivatives penetrated well into cells. In the control group A cells showed strong fluorescence. Control group B showed no fluorescence. See Figure 6 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com