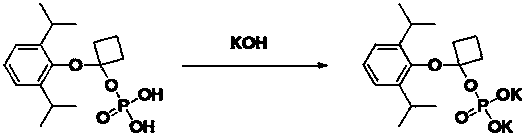
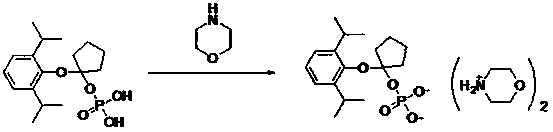
Propofol water-soluble naphthenic phosphate ester derivative
A technology of propofol and bisphosphonic acid, applied in the field of medicinal chemistry, to avoid injection pain and embolism, increase solubility, and reduce side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Preparation of propofol-1-chlorocyclopropyl ether
[0024]
[0025] Under nitrogen protection, sodium hydride (2.5 g, dispersed in mineral oil, 60% by mass, 61.6 mmol) was suspended in anhydrous tetrahydrofuran (100 mL) while stirring, and propofol was added in batches at 0 °C (10.0 g, 56.0 mmol), the resulting mixture was stirred for 10 minutes, and a solution of 1-chloro-1-iodocyclopropane (12.5 g, 61.6 mmol) in tetrahydrofuran (10 mL) was added dropwise. After the dropwise addition was completed, stirring was continued at room temperature for 6 hours. Filter, evaporate solvent under reduced pressure, residue recrystallizes with the mixed solvent of sherwood oil and ethyl acetate (V 石油醚 :V 乙酸乙酯 =6:1), dried to obtain 12.6 g of white solid, yield 89.1%, HPLC purity: 99.4% (254 nm). 1 H NMR (400 MHz, CDCl 3 ( m, 2H).
Embodiment 2
[0026] Embodiment 2: Preparation of O-[1-(propofol-O base)]cycloprop-1-yl monoester phosphoric acid
[0027]
[0028] A mixture of propofol-1-chlorocyclopropyl ether (12.0 g, 47.5 mmol), phosphoric acid (5.6 g, 57.0 mmol), triethylamine (5.8 g, 57.0 mmol), ethanol (100 mL) was heated to 70 °C for 3 hours. Concentrate the solvent under reduced pressure (-0.09 MPa, 40 °C) until white crystals precipitate, then cool with ice-water mixture, filter, and dry to obtain 9.3 g of white solid, yield 62.0%, HPLC purity: 97.6% (254 nm) . 1 H NMR (400MHz, DMSO-d 6 ( m, 2H).
Embodiment 3
[0029] Example 3: Preparation of O-[1-(propofol-O base)]cycloprop-1-yl monoester phosphate disodium
[0030]
[0031] Dissolve O-[1-(propofol-O-yl)]cycloprop-1-yl monoester phosphoric acid (4.5 g, 14.3 mmol) in water (30 mL), add sodium hydroxide (1.15 g, 28.6 mmol). Stirring was continued at room temperature for 1 hour, water was evaporated under reduced pressure, and dried to obtain 5.1 g of white solid, yield 100.0%, HPLC purity: 98.3% (254 nm). 1 H NMR (400MHz, D 2 O) δ: 7.35-7.19 (m, 2H), 6.93-6.62 (m, 1H), 3.20-3.12 (m, 2H), 1.25 (d, 12H), 0.84-0.79 (m, 2H), 0.72-0.66 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com