Method for detecting ochracin A based on fluorescence anisotropy of nucleic acid aptamer
A nucleic acid aptamer and ochratoxin technology, which is applied in fluorescence/phosphorescence, material excitation analysis, etc., can solve problems such as complex methods, and achieve the effects of simple detection process, easy preparation, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
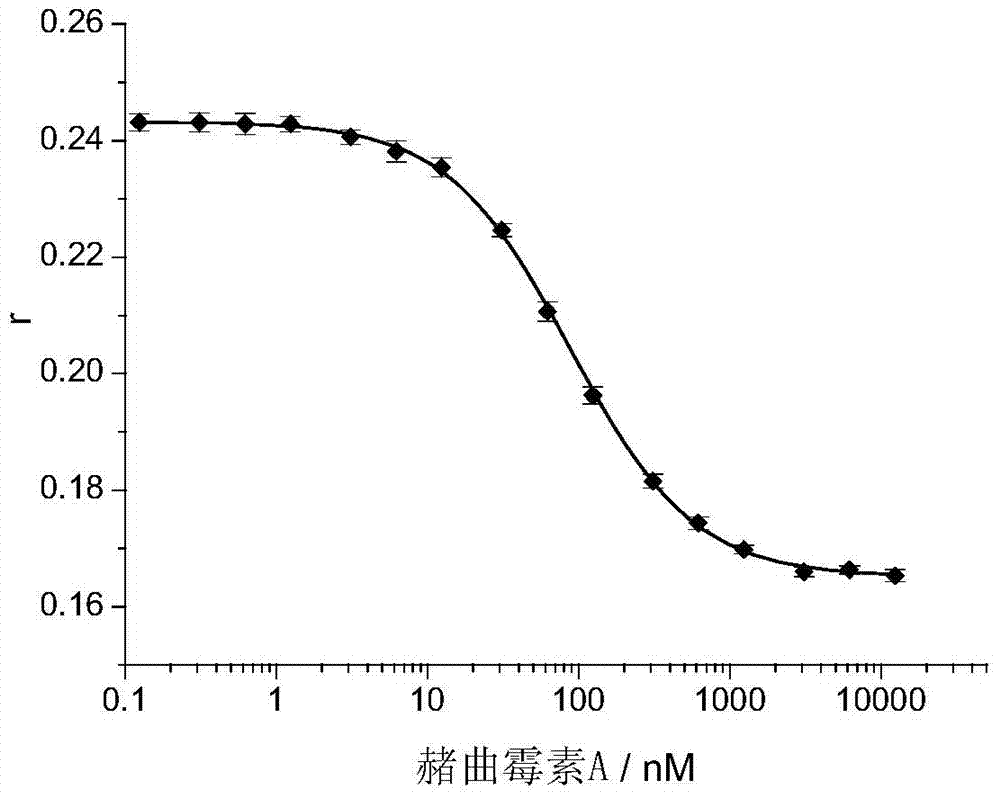
[0015] Example 1: Detection of different concentrations of ochratoxin A ( figure 1 ).
[0016] The nucleic acid aptamer marked by TMR has the following sequence: 5'-GAT CGG GTG TGG GTG GCG TAA AGGGAG CAT CGG ACA-3, TMR is marked on the 10th T base, and the corresponding TMR-labeled aptamer is abbreviated as It is T10-TMR-O36.
[0017] In buffer solution (10mM Tris-HCl, 120mM NaCl, 20mM CaCl 2 , 0.1% Tween20, pH 8.5) mixed T10-TMR-O36 (27nM concentration) with different concentrations of ochratoxin A, incubated at room temperature for 40 minutes, and then measured the fluorescence of T10-TMR-O36 Anisotropy, the excitation light wavelength is 560 nm, and the emission light wavelength is 578 nm. As the concentration of ochratoxin A (Ochratoxin A) increases, the fluorescence anisotropy value r of T10-TMR-O36 gradually decreases, the detection limit is 3nM, the detection range is 3nM to 3μM, and the relative standard deviation of detection is less than 3%.
Embodiment 2
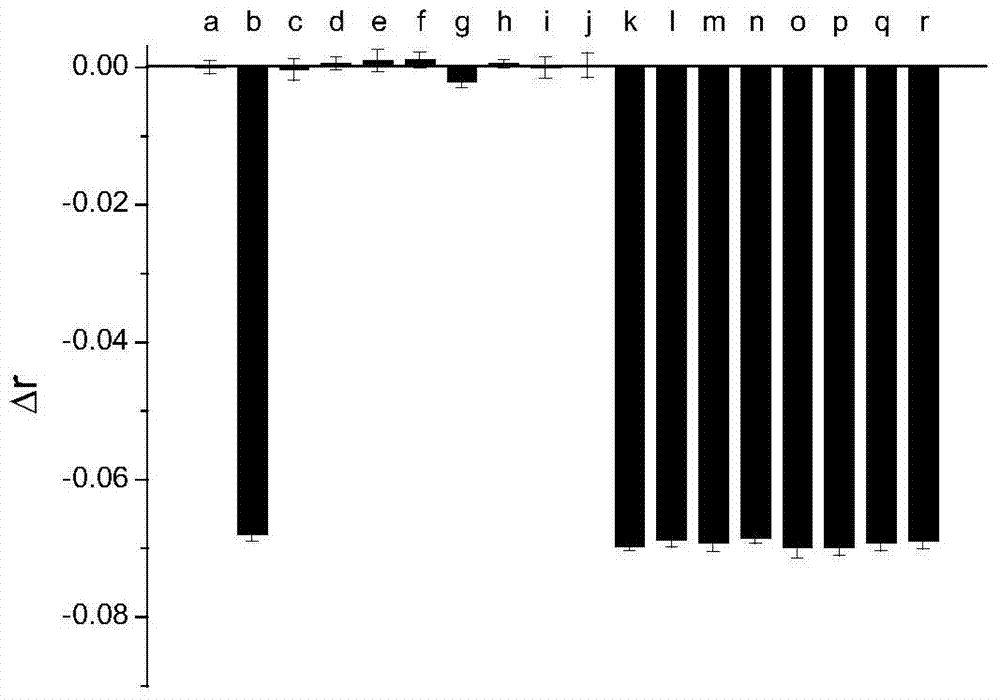
[0018] Example 2: Investigating the selectivity of TMR-labeled nucleic acid aptamer (T10-TMR-O36) in detecting ochratoxin A ( figure 2 ).
[0019] In buffer solution (10mM Tris-HCl, 120mM NaCl, 20mM CaCl 2 , 0.1% Tween20, pH8.5) T10-TMR-O36 (27nM concentration) was mixed with different analytes, incubated at room temperature for 40 minutes, and the fluorescence anisotropy change Δr of T10-TMR-O36 was measured. Only ochratoxin A (620nM) caused a significant decrease in the fluorescence anisotropy value of T10-TMR-O36, and none of the other tested substances could cause a significant change in the fluorescence anisotropy value of T10-TMR-O36. figure 2 In (a) is a buffer solution that does not contain ochratoxin A, (b) is 620nM ochratoxin A, and the tested substances from (c) to (j) are 10μM N-acetyl-L-phenylalanine (NAP ) (c), 7 μM warfarin (d), 9 μM 7-amino-4-methylcoumarin (AMC) (e), 62 μM arginine (f), 62 μM phenylalanine (g), 62 μM aspartic acid (h), 62 μM serine (i), 6...
Embodiment 3
[0020] Example 3: Detection of ochratoxin A ( image 3 ).
[0021] Red wine was diluted 50 times with buffer solution (10mM Tris-HCl, 120mM NaCl, 20mM CaCl2, 0.1%Tween20, pH8.5), and then T10-TMR-O36 (27nM concentration) was mixed with Different concentrations of ochratoxin A were mixed, incubated at room temperature for 40 minutes, and then the fluorescence anisotropy of T10-TMR-O36 was measured, the excitation light wavelength was 560 nm, and the emission light wavelength was 578 nm. As the concentration of ochratoxin A (Ochratoxin A) increased, the fluorescence anisotropy value r of T10-TMR-O36 decreased gradually.
[0022]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com