Metal electrolytic method in alkaline solutions
A technology of alkaline solution and metal electrolysis, which is applied in the direction of photographic process, instrument, photographic auxiliary process, etc., and can solve problems such as rising, steam leakage environment, cost increase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
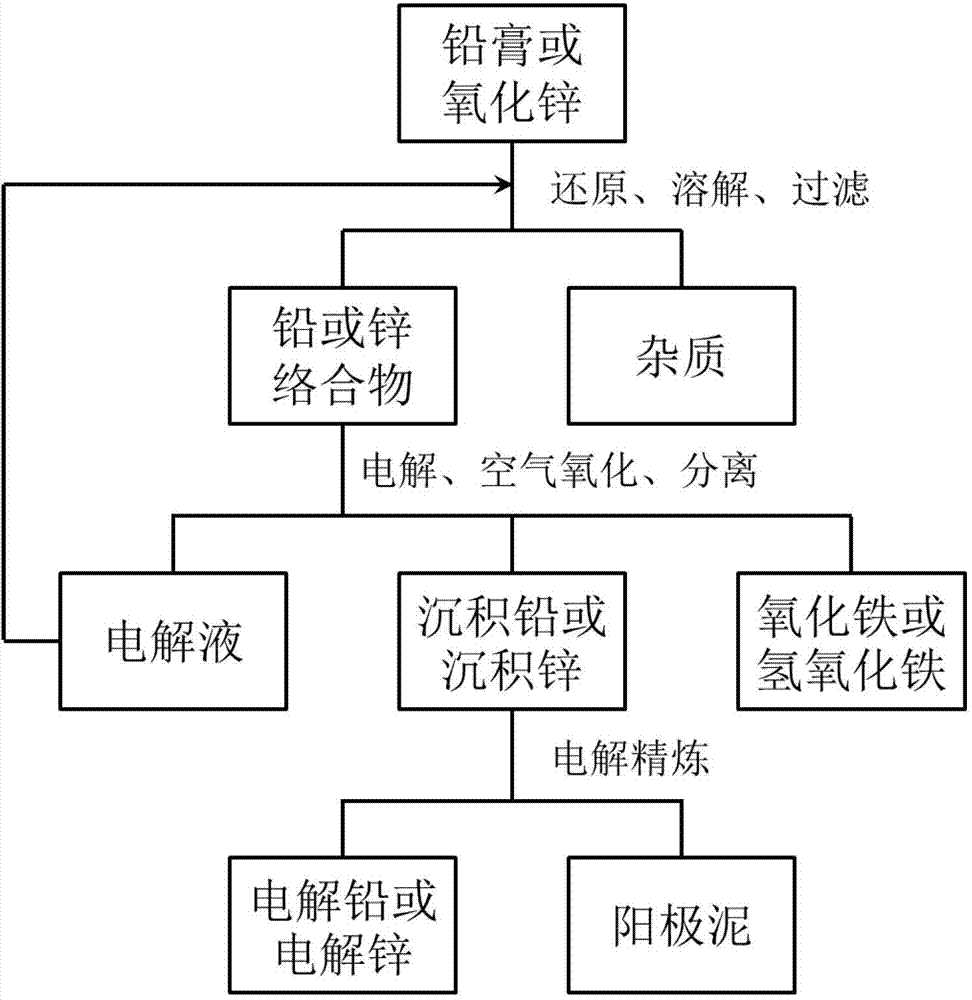
[0079] Take 12V100Ah waste lead-acid battery, break it and separate it to obtain lead paste (lead-containing compound).
[0080] Prepare 10L electrolyte solution containing 0.7mol / L nitrilotriacetic acid, 1mol / L sodium nitrate, and 0.1g / L rosin, and adjust the pH value to 8.0±0.5 with hydrochloric acid-ethanolamine buffer solution. Add 1kg of lead paste and excess lead powder to the electrolyte to dissolve all the lead compounds in the lead paste. The electrolyte is heated to 55°C. The cathode is 40*50*0.1 (length*width*thickness) cm 3 Potassium permanganate passivated stainless steel plate, the anode is 40*50*1 (length*width*thickness) cm 3 Wrought iron plate with a purity of 99%, using a current density of 400A / m 2 Constant current electrolysis for 15 hours, with an average electrolysis voltage of 0.03V. During this period, air was continuously introduced into the solution to oxidize the ferrous complex into iron oxide precipitation, and at the same time, add lead paste t...
Embodiment 2
[0087] Prepare 100L electrolyte solution containing 0.8mol / L phenylalanine, 1mol / L potassium sulfate, and 5g / L gelatin, use disodium hydrogen phosphate-sodium hydroxide solution to adjust the pH value to 13.8±0.2, and add 4kg Ore containing 95% lead oxide. The electrolyte is heated to 70°C. The cathode is 100*100*0.1 (length*width*thickness) cm 3 The lead plate, the anode is 100*100*0.5 (length*width*thickness) cm 3 The purity of the pig iron plate is 95%, and the current density is 600A / m 2 Constant current electrolysis for 5 hours, during which air is continuously introduced into the solution to oxidize the ferrous compound into iron oxide precipitation, and at the same time add lead oxide ore to maintain the lead content in the solution not less than 0.2mol / L, the average electrolysis voltage during the electrolysis process is - 0.05V.
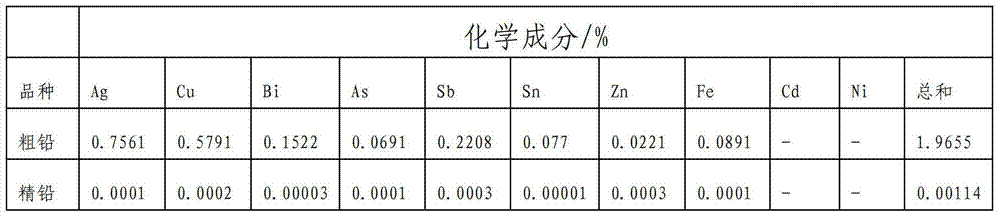
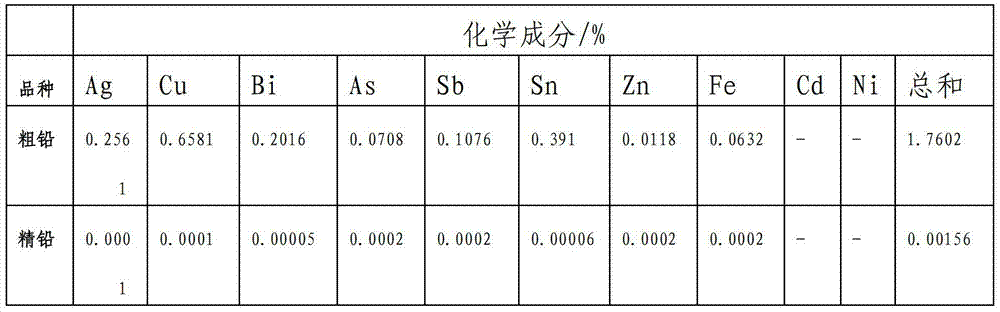
[0088] Cast the rough lead obtained by the above electrolysis into 50*50*2 (length*width*thickness) cm 3 The anode plate, the cathode...
Embodiment 3
[0093] Prepare 500L electrolyte solution containing 1.1mol / L ethylenediamine diacetic acid, 0.5mol / L sodium pyrophosphate, 0.5g / L β-naphthol, and 1g / L bone glue, and use ethylenediamine-hydrochloric acid buffer solution to adjust the pH value to 10.0 ±0.3, add 10kg of ore containing 98% zinc oxide to the electrolyte. The electrolyte is heated to 45°C. The cathode is 200*200*0.1 (length*width*thickness) cm 3 The zinc plate, the anode is 200*200*1.5 (length*width*thickness) cm 3 304 stainless steel plate, using a current density of 200A / m 2 Constant current electrolysis for 48 hours, during which air was continuously introduced into the solution to oxidize the ferrous compound into iron oxide precipitation, and zinc oxide ore was added at the same time to maintain the zinc content in the solution not less than 0.15mol / L, and the average electrolysis voltage during the electrolysis process was 0.51 V.
[0094] Cast the rough zinc obtained by the above electrolysis into 100*10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com