Magnetic pirarubicin nano-drug composite
A magnetic pirarubicin and pirarubicin technology, which can be used in drug combinations, antitumor drugs, pharmaceutical formulations, etc., can solve the problems of inability to withstand perfusion chemotherapy, lack of targeting of chemotherapy drugs, and bladder epithelial cell killing, etc. To achieve the effect of good dispersion and reduce toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of Magnetic THP Nano-Preparation
[0031] 1) Magnetic Fe 3 o 4 Preparation of nanoparticles:
[0032] With reference to the method of J MagnMagn Mater, 2006, 302 (2): 397-404, which is incorporated by reference in its entirety, the preparation of magnetic Fe 3 o 4 Nanoparticles, the specific preparation process is as follows:
[0033] Under nitrogen protection, 8.14 g of ferric chloride hexahydrate and 3.00 g of ferrous chloride tetrahydrate were dissolved in 30 mL of deionized water, and 0.85 mL of 12 mol / L hydrochloric acid solution was added. Add the above solution dropwise to 250 mL of 1.5 mol / L sodium hydroxide solution, stir for 30 min, collect the precipitate, wash with deionized water three times, and dry in vacuum to obtain Fe with an average particle size of about 40 nm. 3 o 4 nanoparticles.
[0034] 2) Aminated Fe 3 o 4 preparation:
[0035] Under the protection of nitrogen, the 0.1g Fe obtained in step 1) 3 o 4 Nanoparticles...
Embodiment 2
[0038] Example 2 Characterization of Magnetic THP Nano-Preparations
[0039] Observation by scanning electron microscopy (Inspect F50, FEI, USA) of magnetic Fe 3 o 4 The shape of nanoparticles, the nanoparticles with a particle size of about 40nm, the particle size distribution is relatively uniform, see Figure 1a .
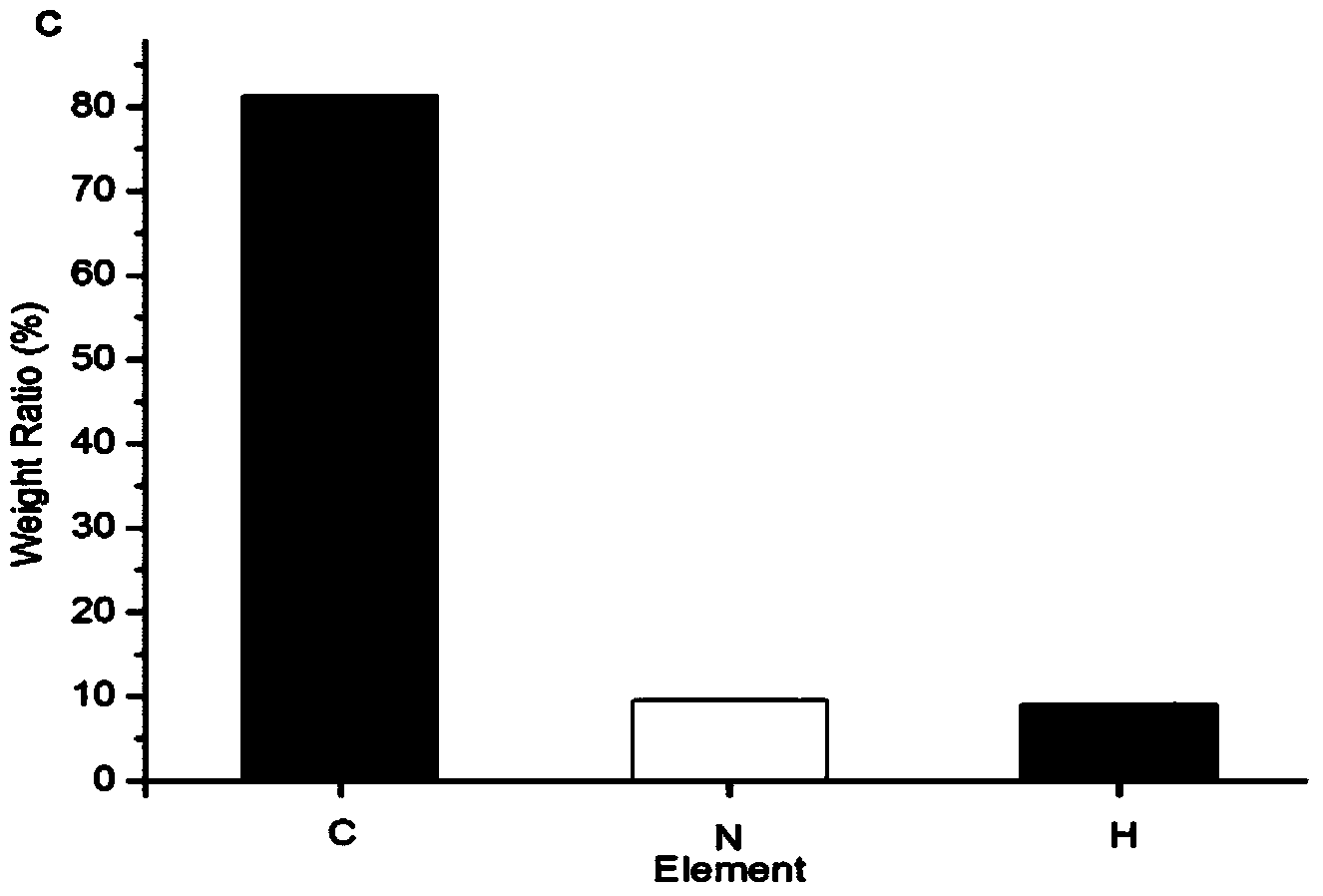
[0040] Aminated Fe was scanned using a Fourier transform infrared spectrometer (Spectrum GX, Perkin Elmer, USA) 3 o 4 ,See Figure 1b 1 ,exist Figure 1b 1 Medium, 3431cm -1 and 1630cm -1 It is the characteristic absorption peak of N-H, which proves that the magnetic nano-Fe 3 o 4 The surface is modified by 3-aminopropyltriethoxysilane, and the surface is rich in active amino groups; determine the infrared spectrum of the magnetic THP nano drug composition, see Figure 1b 2 ,exist Figure 1b 2 Medium, 3000cm -1 Three weaker absorption peaks appear nearby, which are the absorption peaks of C-H on the benzene ring. 1620cm -1 The left and right pea...
Embodiment 3
[0046] Example 3 Determination of Stability of Magnetic THP Nano-Preparations
[0047] Weigh 0.0039g of magnetic THP nano-preparations, ultrasonically disperse them in PBS buffers with pH 7.4, 6.0, and 5.7, place them in dialysis bags, seal them, and immerse the whole in 50mL of PBS buffers with the same pH, at 37°C After standing still at 0.5 h, 1.0 h, 1.5 h, and 2 h, 2 mL of the solution was taken to measure the concentration of THP in it with a UV-Vis spectrophotometer (Lambda900, Perkin Elmer, USA). In addition, under the stirring condition of 700rpm, the THP concentration at the same time point was measured. The drug residue rate was calculated separately.
[0048] Figure 3 shows the THP drug residual rate of magnetic THP nanocomposites in PBS buffer with different pH values under static state and stirred state. The results show that the magnetic THP nano-preparation or composition is pH=7.4 under normal physiological conditions ( Figure 3a ) in the buffer solution,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com