Cyclophosphamide composition freeze-dried powder for injection
A technology of cyclophosphamide and freeze-dried powder injection, which is applied in the direction of freeze-dried delivery, drug combination, powder delivery, etc., can solve the problems of shortening the dissolution time, achieve the effects of improving chemical stability, enhancing activity, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of the freeze-dried powder injection of embodiment 1 injection cyclophosphamide composition (with 2000)
[0028] prescription
[0029] Cyclophosphamide 200g
[0030] Chitosan 100g
[0031] Water for injection 4000mL
[0032] Preparation Process
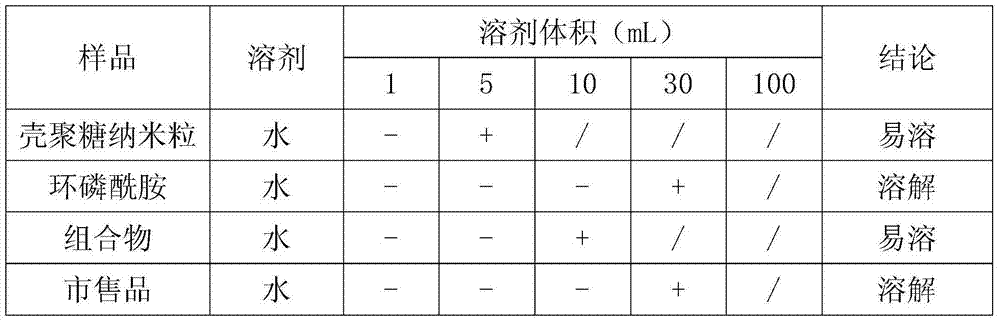
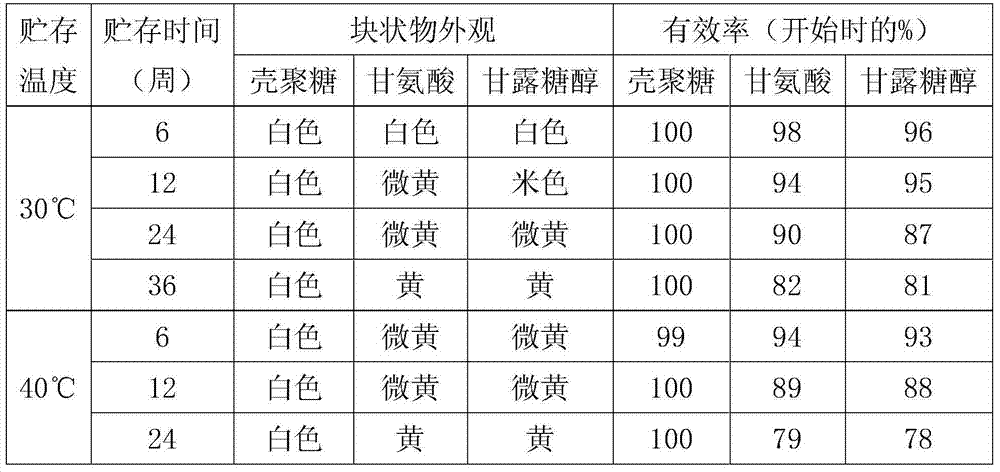
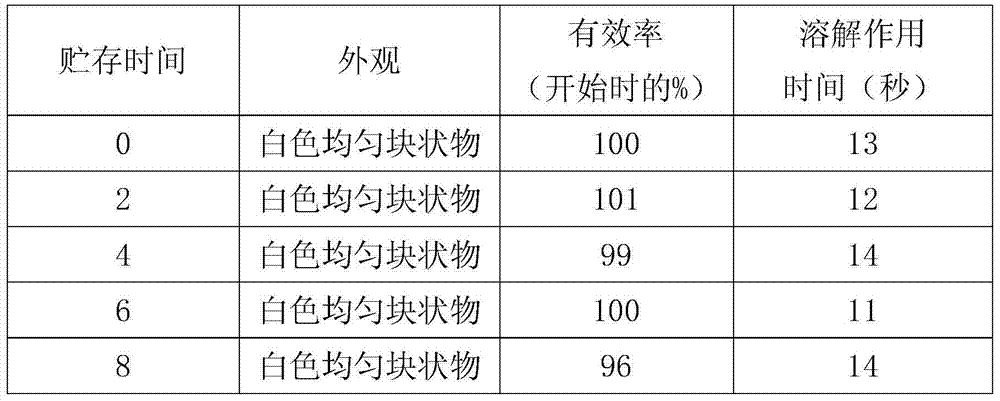
[0033] (1) Weigh 200g of cyclophosphamide and 100g of chitosan nanoparticles, slowly add a certain amount of water for injection cooled to room temperature, and stir until dissolved at 20°C±2°C.
[0034] (2) Weigh 150g of potassium hydroxide to prepare a solution, adjust the pH of the sample solution to 8.0, add water for injection to the resulting solution to the full amount, then add 2g of activated carbon and stir for 30min, filter to remove the activated carbon, and pass the liquid through 0.45μm and 0.22 After the μm microporous membrane is filtered, the content of the intermediate is detected, and the filling amount is calculated as 0.1g (calculated as cyclophosphamide) per bottle.
[0035] (3) Fill a...
Embodiment 2
[0036] The preparation of the freeze-dried powder injection of embodiment 2 injection cyclophosphamide composition (with 2000)
[0037] prescription
[0038] Cyclophosphamide 400g
[0039] Chitosan 200g
[0040] Water for injection 8000mL
[0041] Preparation Process
[0042] (1) Weigh 400g of cyclophosphamide and 200g of chitosan nanoparticles, slowly add a certain amount of water for injection cooled to room temperature, and stir until dissolved at 20°C±2°C.
[0043] (2) Weigh 150g of potassium hydroxide to prepare a solution, adjust the pH of the sample solution to 8.0, add water for injection to the resulting solution to the full amount, then add 4g of activated carbon and stir for 30min, filter to remove the activated carbon, and pass the liquid through 0.45μm and 0.22 After the μm microporous membrane is filtered, the intermediate content is detected again, and the filling amount is calculated as 0.2g (calculated as cyclophosphamide) per bottle.
[0044] (3) Fill ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com