Luminescent material Zn(phen)(HL)2 and preparation method thereof
A technology of znso4 7h2o and complexes, applied in luminescent materials, chemical instruments and methods, zinc organic compounds, etc., can solve the problems of easy weathering, low degree of ligand conjugation, low stability, etc., and achieve the effect of not easy weathering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
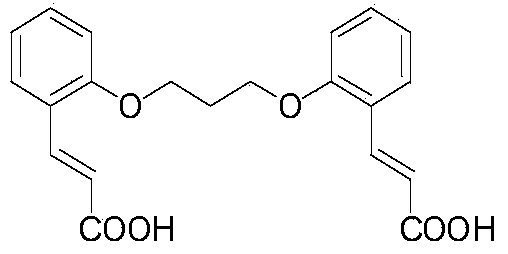
[0027] H used in the present invention 2 The preparation process of L can be found in reference [1].
[0028] The selected ligand H of the present invention 2 The degree of L conjugation is very large. During the reaction H 2 L has taken off an H atom and becomes HL-1 to coordinate with the zinc ion in zinc sulfate. The resulting product is H 2 L is the yield based on calculation. That is, according to the H in the reactant 2 L and Zn(phen)(HL) 2 The molar ratio of Zn(phen)(HL) that should be obtained in theory is converted into 2 quality, and then according to the actual Zn(phen)(HL) 2 The ratio of the latter to the former is the yield.
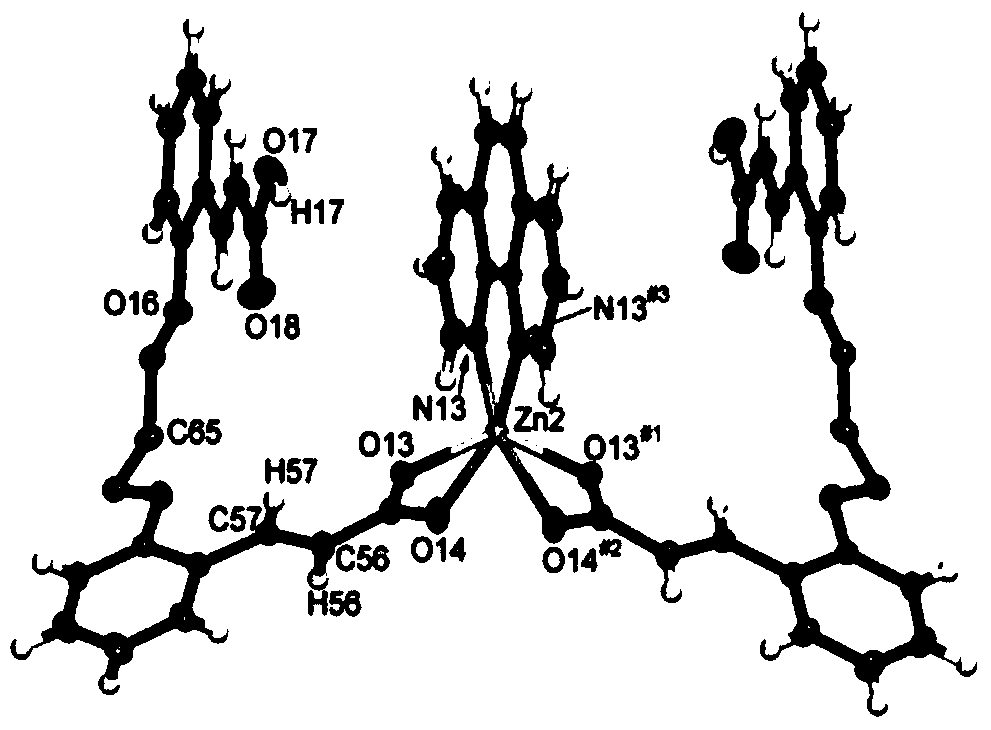
[0029] In the present invention, X-ray diffraction analysis is performed on the final product to obtain its crystal structure. And carry out a series of characterizations on the final product, such as elemental analysis, infrared, fluorescence, x-ray powder diffraction.
[0030] The present invention has also measured the luminous...
Embodiment 1
[0031] The preparation of embodiment 1 complex of the present invention
[0032] Add 35.0 mg (0.095 mmol) H in a 50 mL glass beaker 2 L, 18.0 mg (0.091 mmol) phen, 27.1 mg (0.094 mmol) ZnSO 4 , 1.8mL (23.264 mmol) DMF, 7.8mL (433.333 mmol) H 2 0, the mixture was stirred for 0.5h, then transferred into a 20mL glass bottle with a screw cap, reacted at 80°C for 3 days, cooled to room temperature naturally, and observed pale yellow crystals, which were the target product, which was filtered out from the mother liquor , washed with water and methanol in turn, and then dried naturally.
[0033] ProductZn(phen)(HL) 2 Elemental analysis of C, H, and N in the element, the calculated value (%): C, 66.43; H, 5.38; N, 2.77; the actual measured (%): C, 66.75; H, 4.99; N, 2.86. FT-IR (KBr, cm -1 ): 1635(m), 1599(m), 1555(s), 1406(s), 846(m), 723(s). Note: Elemental analysis values are measured by Perkin-Elmer2400 elemental analyzer; infrared spectrum is measured by NicoletImpact410F...
Embodiment 2
[0037] The preparation of embodiment 2 complexes of the present invention
[0038] In a 50 mL glass beaker was added 36.8 mg (0.1 mmol) of H 2 L, 19.8 mg (0.1 mmol) phen, 28.8 mg (0.1 mmol) ZnSO 4 , 2mL (25.960 mmol) DMF, 8mL (444.444 mmol) H 2 0, the mixture was stirred for 0.5h, then transferred into a 20mL glass bottle with a screw cap, reacted at 80°C for 3 days, cooled to room temperature naturally, and observed pale yellow crystals, which were the target product, which was filtered out from the mother liquor , washed with water and methanol in turn, and then dried naturally.
[0039] The product was characterized by elemental analysis, infrared, fluorescence, and x-ray powder diffraction, and the data obtained were similar to those in Example 1. Illustrate that the crystal structure that makes with embodiment 2 does not change and product is purer (see Figure 6 ), performance also varies for (see Figure 5 ).
[0040] This embodiment is repeated many times, obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com