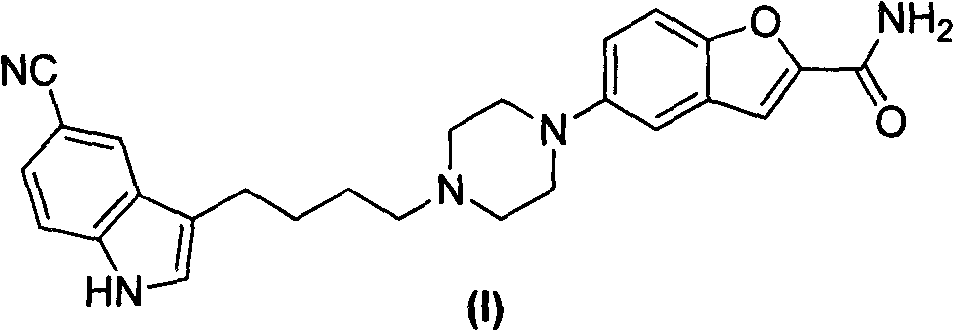
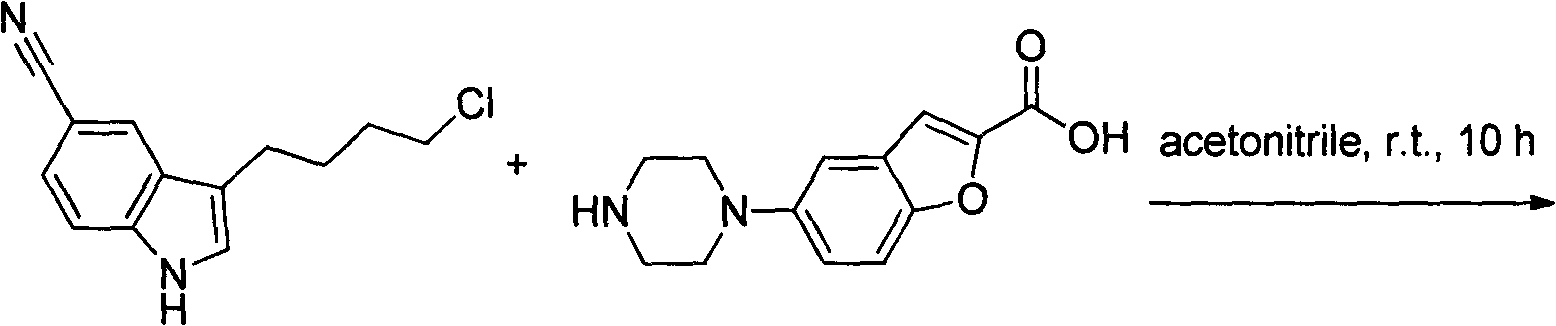
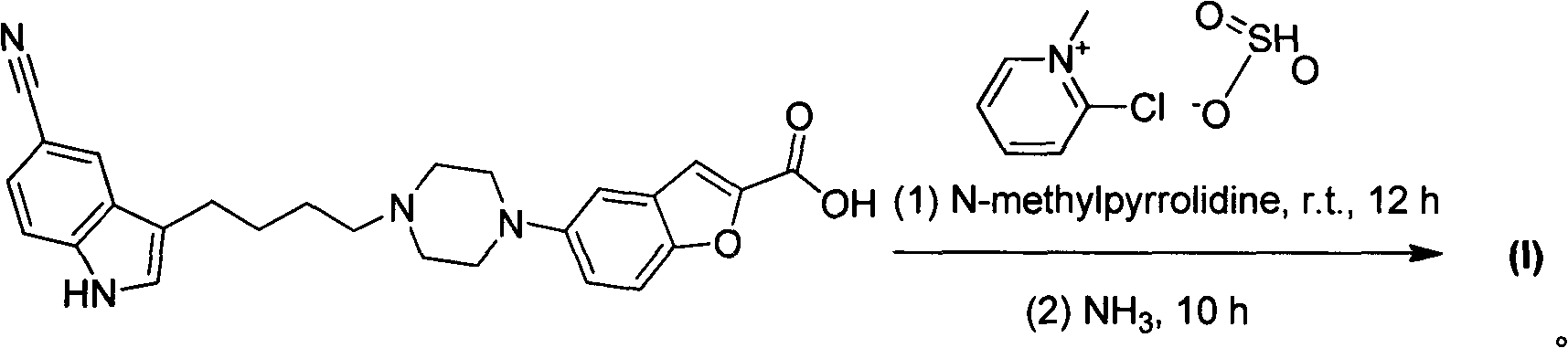
Method for preparing vilazodone
A technology of vilazodone and formate, which is applied in the field of preparation of antidepressant vilazodone, can solve the problems of harmful organic transition metal catalysts and expensive environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 150 grams of 5-nitrosalicylaldehyde are added to the reaction flask, then 300 grams of potassium bicarbonate and 1000 milliliters of ethanol are added, and stirred evenly; 236 grams of diethyl bromomalonate are added, heated and refluxed overnight; TLC tracks to The reaction was basically completed, cooled to room temperature, filtered, the filter cake was washed with 900 mL of water, and the filter cake was taken out and dried to obtain 139 grams of 5-nitrophenylpropane-2-carboxylate (III), with a yield of 66% and a purity of 99.3 %.
Embodiment 2
[0036] The preparation of embodiment two 5-aminobenzofuran-2-ethyl carboxylate (IV)
[0037] 100 grams of 5-nitrobenzofuran-2-carboxylate (III), 10 grams of 7% palladium / carbon, and 800 milliliters of ethanol were successively added to the reaction flask, and after hydrogen replacement for 3 times, they were stirred at room temperature and tracked by TLC. until the reaction is complete. After filtration, the filtrate was concentrated directly to obtain 85 g of ethyl 5-aminobenzofuran-2-carboxylate (IV) as an oil, which was directly used in the next reaction. The preparation of embodiment three 5-piperazinyl benzofuran-2-ethyl carboxylate (V)
Embodiment 3
[0038] 119 grams of ethyl 5-aminobenzofuran-2-carboxylate (IV) is added in the reaction flask, 260 grams of two (2-chloroethylamine) hydrochloride and 2500 milliliters of ethanol are added, then 160 grams of potassium carbonate are added ; Protected after nitrogen replacement, heated to reflux overnight. HPLC traces the reaction until the remaining ≤ 7% of the raw material is the end point of the reaction; the reaction solution is cooled to room temperature, filtered, the filter cake is washed with water, and the filter cake is taken out and dried to obtain 80 grams of off-white solid 5-piperazinylphenylpropane-2-carboxylic acid Ethyl ester (V), yield 50%, purity 98.5%. The preparation of embodiment four 5-piperazinyl benzofuran-2-carboxamide (VI)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com