Gastrodin injection and preparation process thereof
A technology for gastrodin injection and gastrodin, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, cardiovascular system diseases, etc., and can solve adverse reactions, increase risks, and instability of gastrodin aqueous solution. and other problems, to achieve the effect of stable gastrodin content and high clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
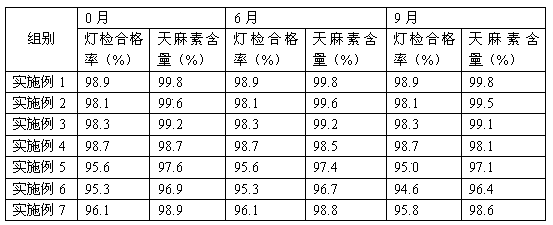
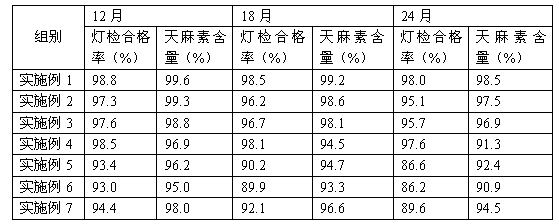
Examples
Embodiment 1
[0014] Embodiment 1: a kind of gastrodin injection, comprises the raw material of following weight ratio: gastrodin 100g, lactose 0.8g, procaine hydrochloride 1.6g, sodium thiosulfate 0.5g, nicotinamide 2.6g, glycine 0.7g, water for injection 2000ml;
[0015] The preparation process of the present invention is as follows:
[0016] 1) Weigh the amount of gastrodin, add 80% of the total amount of water for injection, dissolve the drug in an ultrasonic dissolver, stir evenly, take sodium hydroxide, adjust the pH value of the liquid to 6.0, and then add lactose to obtain solution A;
[0017] 2) Take 15% of the total amount of water for injection, add procaine hydrochloride, sodium thiosulfate, nicotinamide and glycine in sequence, stir well to obtain solution B;
[0018] 3) Mix solution A and solution B, stir evenly, and then add water for injection to a sufficient amount; take a sample for inspection, measure pH and content, after passing the test, the drug solution is reduced b...
Embodiment 2
[0019] Embodiment 2: A kind of gastrodin injection comprises the following raw and auxiliary materials of weight ratio: gastrodin 100g, lactose 0.2g, procaine hydrochloride 0.9g, sodium thiosulfate 0.1g, nicotinamide 2.2g, glycine 0.4 g. Water for injection 2000ml;
[0020] The preparation process of the present invention is as follows:
[0021] 1) Weigh the amount of gastrodin, add 80% of the total amount of water for injection, dissolve the drug in an ultrasonic dissolver, stir evenly, take sodium hydroxide, adjust the pH value of the liquid to 5.0, and then add lactose to obtain solution A;
[0022] 2) Take 15% of the total amount of water for injection, add procaine hydrochloride, sodium thiosulfate, nicotinamide and glycine in sequence, stir well to obtain solution B;
[0023] 3) Mix solution A and solution B, stir evenly, and then add water for injection to a sufficient amount; take a sample for inspection, measure pH and content, after passing the test, the drug soluti...
Embodiment 3
[0024] Embodiment 3: A kind of gastrodin injection, including the following raw materials and auxiliary materials in weight ratio: gastrodin 100g, lactose 1.3g, procaine hydrochloride 2.1g, sodium thiosulfate 0.8g, nicotinamide 3.1g, glycine 1.1g, water for injection 2000ml;
[0025] The preparation process of the present invention is as follows:
[0026] 1) Weigh the amount of gastrodin, add 80% of the total amount of water for injection, dissolve the drug in an ultrasonic dissolver, stir evenly, take sodium hydroxide, adjust the pH value of the liquid to 7.0, and then add lactose to obtain solution A;
[0027] 2) Take 15% of the total amount of water for injection, add procaine hydrochloride, sodium thiosulfate, nicotinamide and glycine in sequence, stir well to obtain solution B;
[0028] 3) Mix solution A and solution B, stir evenly, and then add water for injection to a sufficient amount; take a sample for inspection, measure pH and content, after passing the test, the d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com