Gastrodin injection and preparation process thereof
A technology of gastrodin injection and gastrodin, which can be applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, cardiovascular system diseases, etc., and can solve problems such as adverse reactions, increased risks, and granulation formation. To achieve the effect of stable gastrodin content and high clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
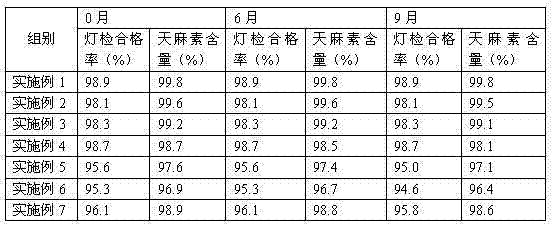
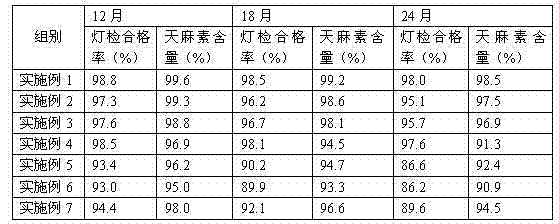
Image
Examples
Embodiment 1
[0014] Example 1: A gastrodin injection comprising the following raw materials in weight ratio: gastrodin 100g, lactose 0.8g, procaine hydrochloride 1.6g, sodium thiosulfate 0.5g, nicotinamide 2.6g, glycine 0.7g, 2000ml water for injection;
[0015] The preparation process of the present invention is as follows:
[0016] 1) Weigh the dosage of gastrodin, add 80% of the total amount of water for injection, dissolve the drug in an ultrasonic dissolver, stir evenly, take sodium hydroxide, adjust the PH value of the drug solution to 6.0, and then add lactose to obtain solution A;
[0017] 2) Take 15% of the total amount of water for injection, add procaine hydrochloride, sodium thiosulfate, nicotinamide and glycine in sequence, stir evenly to obtain solution B;
[0018] 3) Mix solution A and solution B, stir well, and then add water for injection to a sufficient amount; take samples and send for inspection, determine pH and content, after passing the drug solution through 0.22μm micropore...
Embodiment 2
[0019] Example 2: A gastrodin injection including the following raw materials in weight ratio: gastrodin 100g, lactose 0.2g, procaine hydrochloride 0.9g, sodium thiosulfate 0.1g, nicotinamide 2.2g, and glycine 0.4 g. 2000ml water for injection;
[0020] The preparation process of the present invention is as follows:
[0021] 1) Weigh the dosage of gastrodin, add 80% of the total amount of water for injection, dissolve the drug in an ultrasonic dissolver, stir evenly, take sodium hydroxide, adjust the PH value of the drug solution to 5.0, and then add lactose to obtain solution A;
[0022] 2) Take 15% of the total amount of water for injection, add procaine hydrochloride, sodium thiosulfate, nicotinamide and glycine in sequence, stir evenly to obtain solution B;
[0023] 3) Mix solution A and solution B, stir well, and then add water for injection to a sufficient amount; take samples and send for inspection, determine pH and content, after passing the drug solution through 0.22μm micro...
Embodiment 3
[0024] Example 3: A gastrodin injection comprising the following raw materials in weight ratio: gastrodin 100g, lactose 1.3g, procaine hydrochloride 2.1g, sodium thiosulfate 0.8g, nicotinamide 3.1g, glycine 1.1g, 2000ml water for injection;
[0025] The preparation process of the present invention is as follows:
[0026] 1) Weigh the dosage of gastrodin, add 80% of the total amount of water for injection, dissolve the drug in an ultrasonic dissolver, stir evenly, take sodium hydroxide, adjust the pH of the drug solution to 7.0, and then add lactose to obtain solution A;
[0027] 2) Take 15% of the total amount of water for injection, add procaine hydrochloride, sodium thiosulfate, nicotinamide and glycine in sequence, stir evenly to obtain solution B;
[0028] 3) Mix solution A and solution B, stir well, and then add water for injection to a sufficient amount; take samples and send for inspection, determine pH and content, after passing the drug solution through 0.22μm micropores, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com