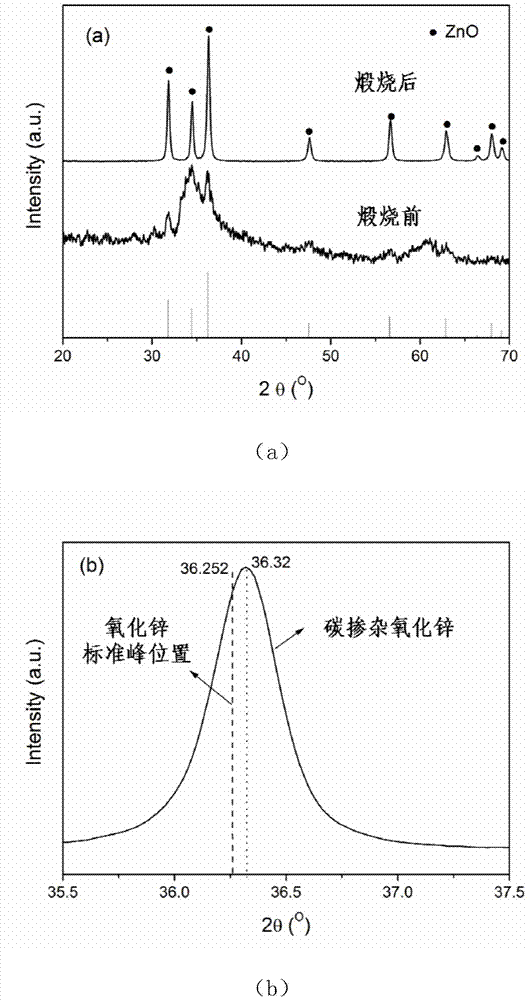
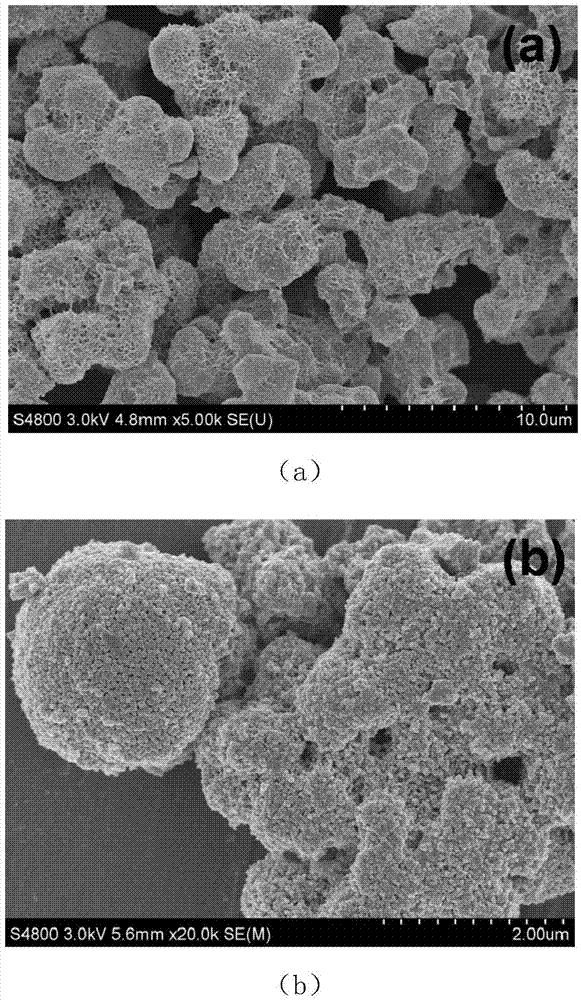
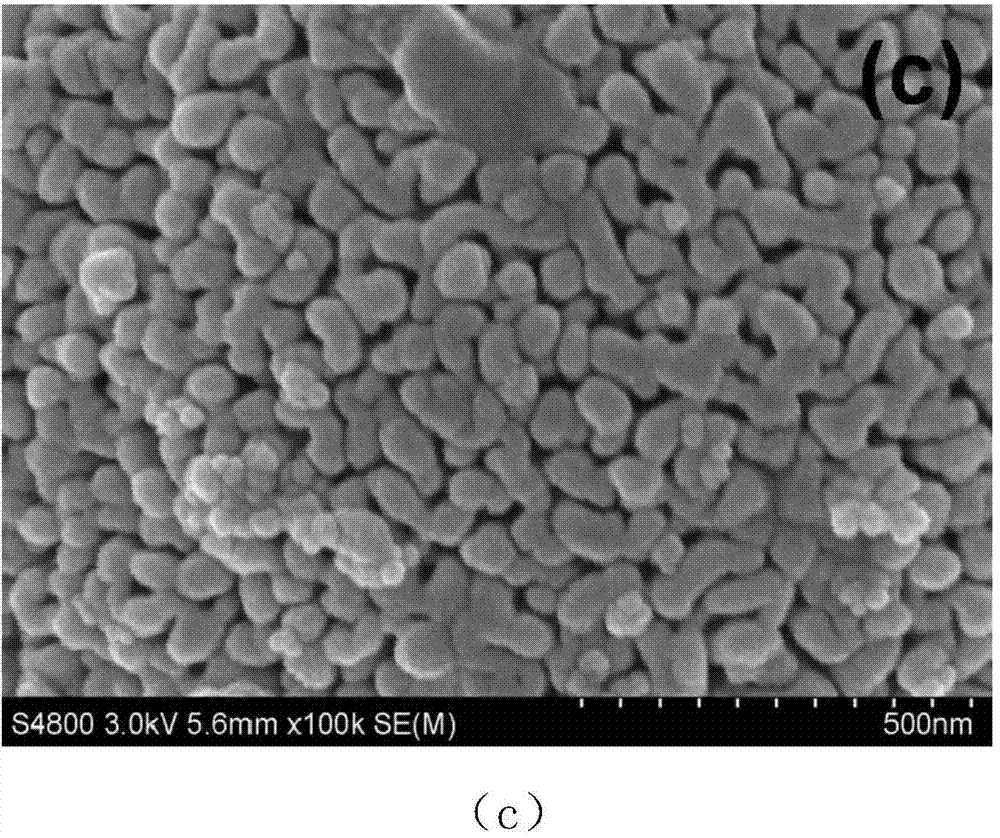
Method for preparing carbon doped zinc oxide
A technology of zinc oxide and zinc gluconate, applied in the direction of zinc oxide/zinc hydroxide, etc., can solve the problems of harsh conditions, complicated process, unfavorable industrialization, etc., and achieve the effect of short reaction time, good repeatability and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) Dissolve zinc gluconate in water to obtain a zinc gluconate solution, and then use 0.2mol / L NaOH solution as a pH regulator to adjust the pH of the zinc gluconate solution to 6; among them, the gluconic acid in the zinc gluconate solution The concentration of zinc is 0.1mol / L;
[0023] 2) Add the pH-adjusted zinc gluconate solution into a microwave digestion tank, then move the microwave digestion tank into a microwave hydrothermal synthesizer, perform a synthesis reaction at 180°C for 3 hours, and then cool naturally to room temperature to obtain the product;
[0024] 3) Centrifuge the product to collect the solid, then wash the solid with deionized water and absolute ethanol, and dry it to obtain a brown zinc oxide precursor;
[0025] 4) The product was calcined in an air atmosphere at 200°C for 2 hours, and cooled naturally to room temperature to obtain carbon-doped zinc oxide.
[0026] The visible light absorption intensity of the carbon-doped zinc oxide obtaine...
Embodiment 2
[0028] 1) Dissolve zinc gluconate in water to obtain a zinc gluconate solution, and then use 1mol / L NaOH solution as a pH regulator to adjust the pH of the zinc gluconate solution to 8; among them, zinc gluconate in the zinc gluconate solution The concentration is 1mol / L;
[0029] 2) Add the pH-adjusted zinc gluconate solution into a microwave digestion tank, then move the microwave digestion tank into a microwave hydrothermal synthesizer, perform a synthesis reaction at 250°C for 1 hour, and then cool naturally to room temperature to obtain the product;
[0030] 3) Centrifuge the product to collect the solid, then wash the solid with deionized water and absolute ethanol, and dry it to obtain a brown zinc oxide precursor;
[0031] 4) The product was calcined at 400°C in an air atmosphere for 0.5 hours, and cooled naturally to room temperature to obtain carbon-doped zinc oxide.
[0032] The visible light absorption intensity of the carbon-doped zinc oxide obtained in this exam...
Embodiment 3
[0034] 1) Dissolve zinc gluconate in water to obtain a zinc gluconate solution, and then use 0.5mol / L NaOH solution as a pH regulator to adjust the pH of the zinc gluconate solution to 7; among them, the gluconic acid in the zinc gluconate solution The concentration of zinc is 0.5mol / L;
[0035] 2) Add the pH-adjusted zinc gluconate solution into a microwave digestion tank, then move the microwave digestion tank into a microwave hydrothermal synthesizer, perform a synthesis reaction at 200°C for 2 hours, and then cool naturally to room temperature to obtain the product;
[0036] 3) Centrifuge the product to collect the solid, then wash the solid with deionized water and absolute ethanol, and dry it to obtain a brown zinc oxide precursor;
[0037] 4) The product was calcined in an air atmosphere at 300°C for 1 hour, and cooled naturally to room temperature to obtain carbon-doped zinc oxide.
[0038] The visible light absorption intensity of the carbon-doped zinc oxide obtained...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com