Mixture of poly-pneumococcal capsular polysaccharide-protein conjugates and preparation method of mixture
A technology of pneumococcus and capsular polysaccharide, which is applied in the direction of botany equipment and methods, biochemical equipment and methods, medical preparations of non-active ingredients, etc., can solve the problem of inability to reduce the cost of vaccine production, short retention time, and ineffectiveness Protect the body from bacterial invasion and other issues, and achieve the effects of low preparation cost, good immunogenicity, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
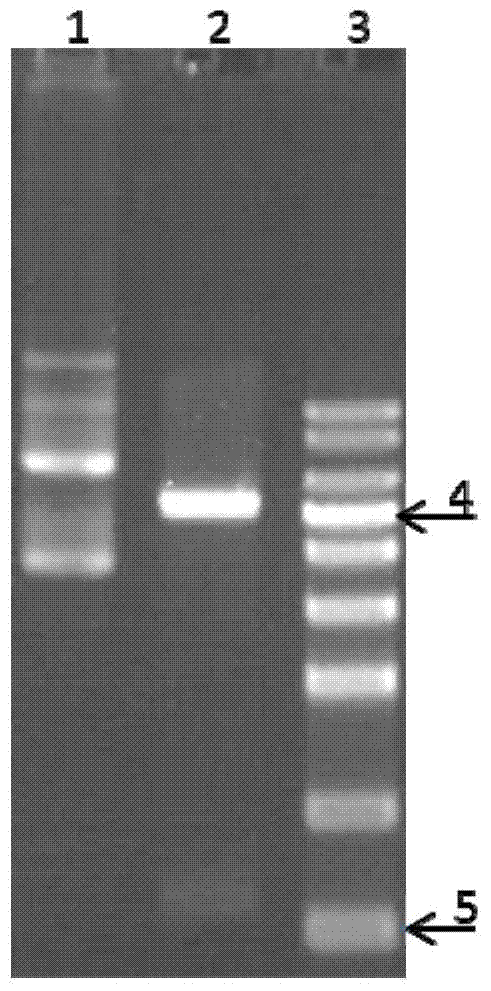
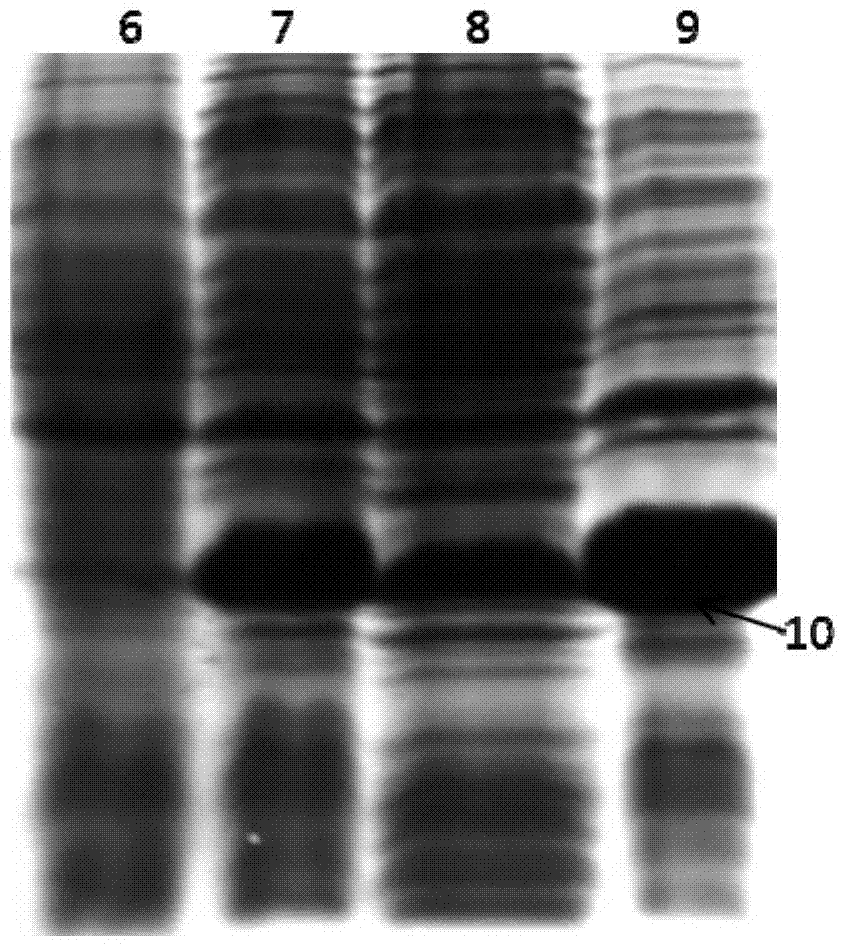
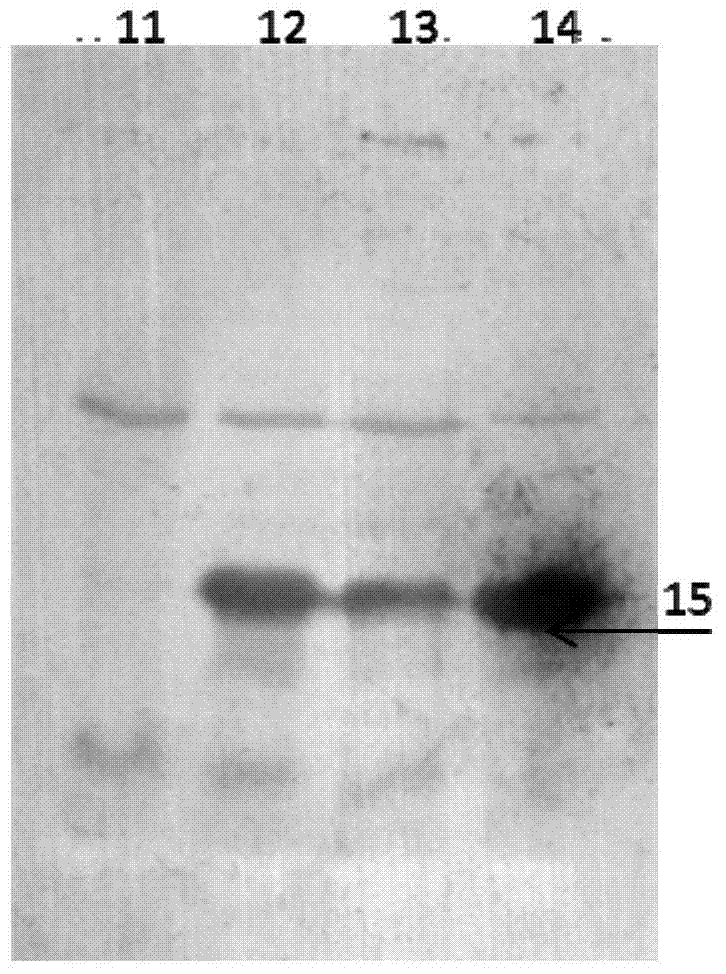
Image
Examples
Embodiment 1
[0047] Example 1: A mixture of multiple pneumococcal capsular polysaccharide-protein conjugates, comprising 13 pneumococcal capsular polysaccharide-protein conjugates and aluminum phosphate adjuvant, each pneumococcal capsular polysaccharide-protein conjugate The material is the combination of corresponding serotype pneumococcal capsular polysaccharides with the same protein carrier through covalent bonds, and the 13 kinds of pneumococcal capsular polysaccharides are derived from pneumococcal serotypes 1, 3, 4, 5, 6A, 6B , 7F, 9V, 14, 18C, 19A, 19F and 23F were purified and extracted from the bacterial culture fluid, and the protein carrier was the A chain protein of the diphtheria toxin mutant CRM197 obtained by gene recombinant Escherichia coli expression, the gene recombinant The nucleotide sequence of the A chain protein of diphtheria toxin mutant CRM197 is:
[0048] 1 GGCGCTGACG ATGTTGTTGA CTCCTCGAAA TCCTTTGTTA TGGAAAACTT CTCCTCCTAC
[0049] 61 CACGGCACGA AACCGGGCTA T...
Embodiment 2
[0058] Embodiment 2: Change the adjuvant in embodiment 1 to aluminum hydroxide.
[0059] The preparation method of the mixture of the multivariate pneumococcal capsular polysaccharide-protein conjugate described in Example 1 is as follows:
[0060] First, the preparation of the A-chain protein of the diphtheria toxin mutant CRM197
[0061] 1. Confirmation of amino acid sequence of A chain of diphtheria toxin mutant CRM197 protein
[0062] The diphtheria toxin mutant CRM197 protein A chain expressed by genetic recombination is composed of amino acid polypeptides from 1 to 193 in the amino acid sequence of diphtheria toxin. The complete gene sequence of CRM197 (HW71379) was obtained from GenBank, and its amino acid sequence is as follows:
[0063] 1 GADDVVDSSK SFVMENFSSY HGTKPGYVDS IQKGIQKPKS GTQGNYDDDW KEFYSTDNKY
[0064] 61 DAAGYSVDNE NPLSGKAGGV VKVTYPGLTK VLALKVDNAE TIKKELGLSL TEPLMEQVGT
[0065] 121 EEFIKRFGDG ASRVVLSLPF AEGSSSVEYI NNWEQAKALS VELEINFETR GKRGQDAMYE
[006...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com