A kind of zl006 liposome and preparation method thereof
A technology of liposomes and lipid bilayers, which is applied in the directions of liposome delivery, pharmaceutical formulations, and medical preparations of inactive ingredients, etc., can solve the problems of limited ability to penetrate the blood-brain barrier and limited therapeutic effects, To achieve the effect of simple and reliable preparation method, stable quality and clear appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
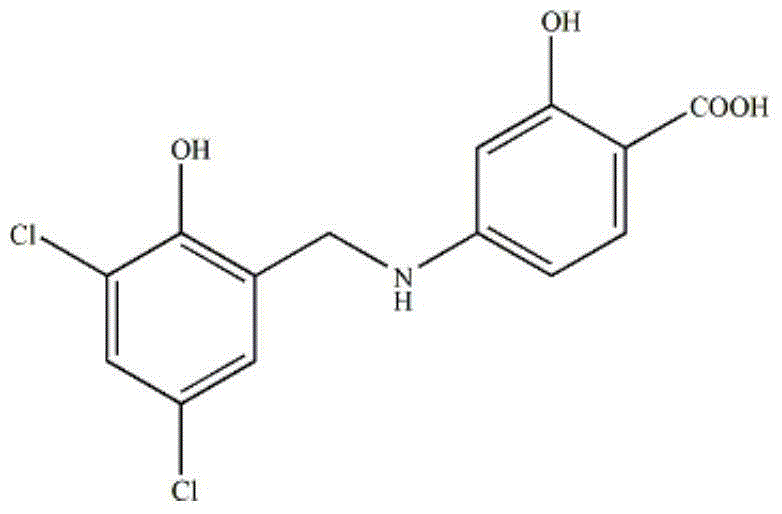
[0031] Embodiment 1: the preparation of ZL006 liposome
[0032] Using the ethanol injection method, accurately weigh 16.7mg of ZL006, 80mg of phospholipids (the phospholipids in the content of the invention can be used, soybean phospholipids are easier to obtain), 20mg of cholesterol, add 7.5mL of absolute ethanol, vortex to dissolve and mix evenly to obtain Organic phase; add the organic phase dropwise to the continuously stirring water phase at a temperature of 50°C and a stirring speed of 550rpm, wherein the water phase is an acetic acid-sodium acetate buffer solution with a pH of 4.0 and a concentration of 0.1mol / L; drop Continue to stir for 10 minutes under the same conditions after the addition is complete; then use an ultrasonic cell pulverizer to reduce the particle size, the ultrasonic conditions are ultrasonic power 475W, intermittent ultrasonic, ultrasonic 2s stop 3s, ultrasonic 5min in total; then reduce the organic solvent by rotary evaporation under reduced pressu...
Embodiment 2
[0035] Embodiment 2: the quality evaluation of ZL006 liposome
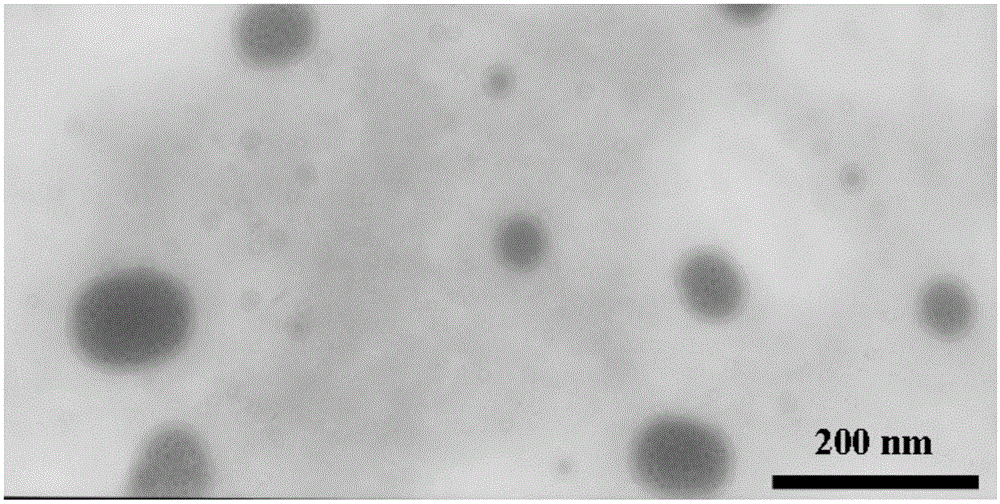
[0036] Appearance of liposome: relatively clear with blue opalescence.
[0037] Encapsulation efficiency (EE%) and drug loading (DL%) determination.
[0038] (1) HPLC method to establish ZL006 standard curve:
[0039] Chromatographic conditions, chromatographic column: Hanbon Phecda C18 (4.6mm×150mm, 5μm; Jiangsu Hanbon Technology Co., Ltd.); mobile phase: methanol-0.25mol / L pH6.0 acetate buffer (65:35; v / v ); Flow rate: 1.0ml / min; UV detection wavelength: 284nm; Column temperature: 30°C; Injection volume: 20μL.
[0040]Standard curve drawing: Accurately weigh 0.0252g of ZL006 dried at 105°C to constant weight in a 50mL volumetric flask, and dilute to the mark with mobile phase to obtain a standard stock solution with a concentration of about 504μg / mL. Precisely pipette 0.05, 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, 8.0, 10.0mL of a series of stock solution into a 50mL volumetric flask, and dilute the mobile phase to the ...
Embodiment 3
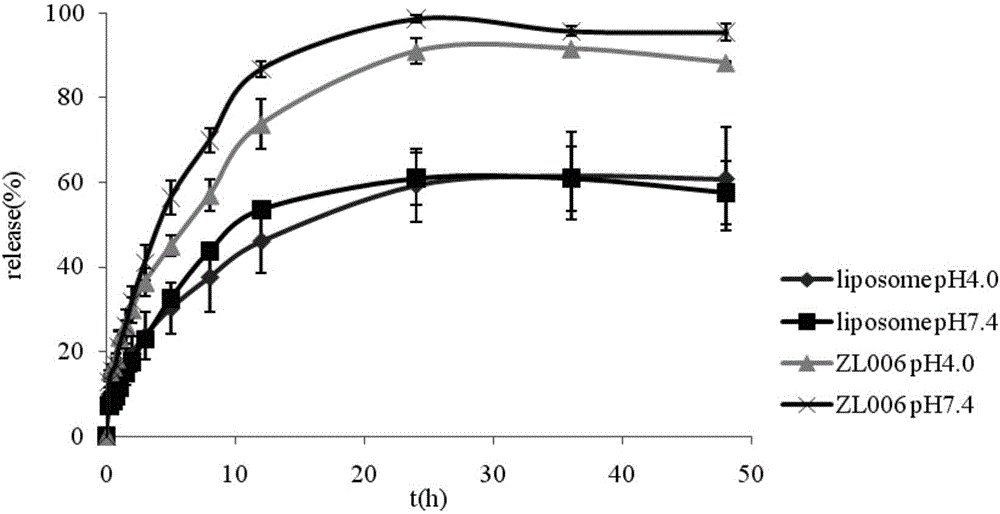
[0050] Example 3: In vitro release of ZL006 liposomes
[0051] Precisely pipette 2mL of the prepared ZL006 liposome (containing about 2.5mg of ZL006) into the treated dialysis bag, tie both ends with a rope and put it into 50mL of release medium (add 0.5% Tween 80 acetate Buffer solution, pH 4.0 and pH 7.4, two parallel groups for each pH) Erlenmeyer flasks, shaking at a constant temperature at 37°C on a shaker, with a rotation speed of 160rpm, at 0, 0.25, 0.5, 0.75, 1, 1.5 , 2, 3, 5, 8, 12, 24, 36, and 48 h to take 0.5 mL of medium, and at the same time add fresh release medium of the same temperature and volume. After the medium taken out was filtered through a 0.22 μm microporous membrane, it was determined by HPLC.
[0052] Calculation of cumulative release (Q n ):
[0053] Q n = C n × V 0 + Σ i ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com