Novel bi-carbodiimide compound and preparation method thereof
A biscarbodiimide compound technology, applied in the field of new biscarbodiimide compounds and their preparation, can solve problems such as inconvenient use, increased operating burden, and difficult crystallization and purification, and achieve easy crystallization and purification, and improve Anti-hydrolysis performance, the effect of convenient processing operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
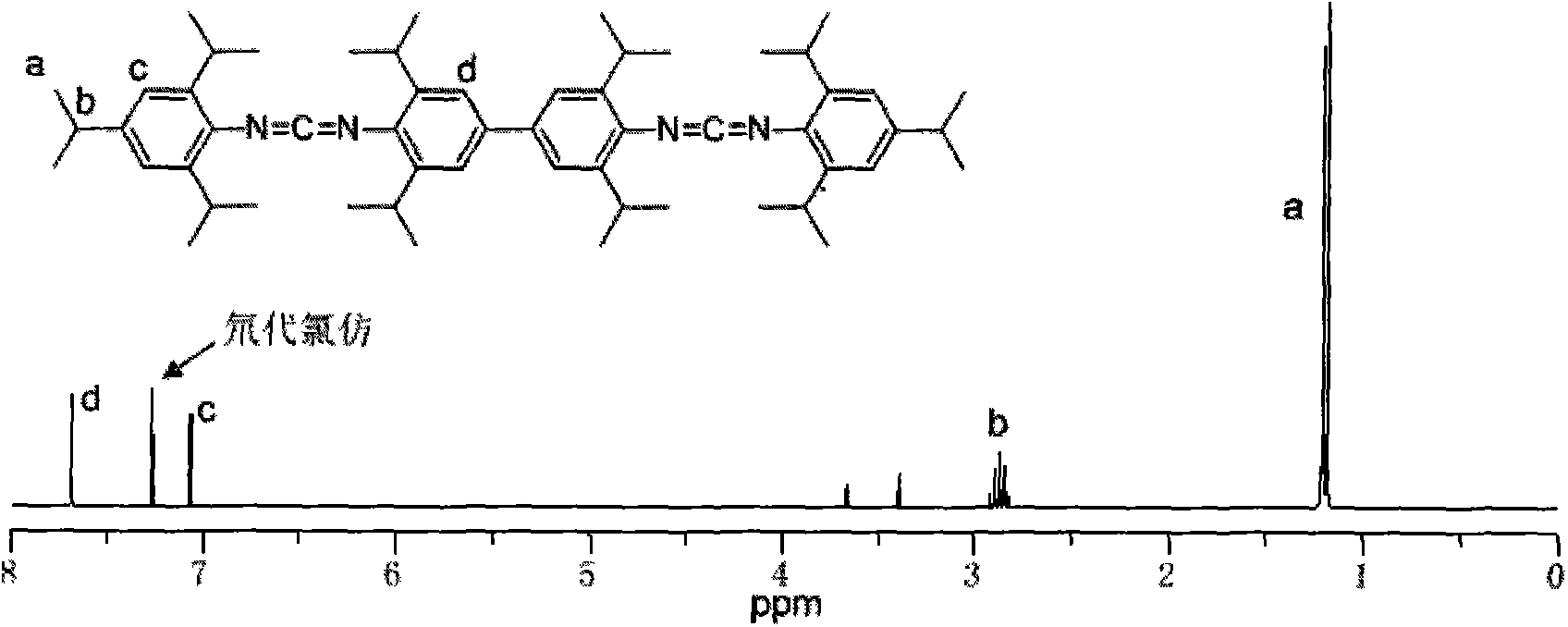
[0032] Add 3.54kg3,3',5,5'-tetraisopropylbiphenyldiamine and 5.22kg2,4,6-triisopropylphenyl isothiocyanate into the reactor, then inject 40L of toluene, Stir mechanically and mix evenly, then heat up to 90°C for addition reaction, keep warm for 8 hours until it is completely converted into dithiourea intermediate; add 4kg of 30%wt sodium hydroxide aqueous solution, and add 59.6kg of 10% sodium hydroxide dropwise within 2h under stirring at 30°C wt sodium hypochlorite aqueous solution, control the rate of addition so that the temperature does not rush to 60°C, react at 40°C for 3 hours after the drop is complete, and end the reaction. Washing, suction filtration removes impurity, filtrate layering, leaves and takes organic layer, obtains crude product after decompression distillation reclaims organic solvent, and crude product recrystallizes with 40L methanol, obtains 7.3kg white crystal biscarbodiimide compound (I -1),
[0033] Yield 90.57%;
Embodiment 2
[0035] Add 3.54kg3,3',5,5'-tetraisopropylbiphenyldiamine and 6.06kg2,4,6-tri-tert-butylphenyl isothiocyanate into the reactor, then inject 45L of toluene, Stir mechanically and mix evenly, then heat up to 90°C for addition reaction, keep warm for 10 hours until it is completely converted into dithiourea intermediate; add 4kg of 30%wt sodium hydroxide aqueous solution, and add 52.8kg of 10% sodium hydroxide dropwise within 2h under stirring at 30°C wt sodium hypochlorite aqueous solution, control the rate of addition so that the temperature does not rush to 60°C, react at 40°C for 4 hours after the drop is complete, and end the reaction. Washing, suction filtration to remove impurities, filtrate layering, leave the organic layer, obtain the crude product after decompression distillation reclaims the organic solvent, the crude product is recrystallized with 45L methanol, obtains 7.9kg white crystal biscarbodiimide compound (I -2), yield 88.76%;
Embodiment 3
[0037] Add 2.4kg3,3',5,5'-tetramethylbenzidinediamine and 5.22kg2,4,6-triisopropylphenyl isothiocyanate into the reaction kettle, then inject 30L dichloroethylene Alkanes, mechanically stirred and mixed evenly, then heated up to 70°C for addition reaction, kept the temperature for 3 hours until it was completely converted into dithiourea intermediate; added 3.5kg of 30%wt sodium carbonate aqueous solution, stirred at 30°C, and added dropwise 45.3 kg10%wt sodium hypochlorite aqueous solution, control the rate of addition so that the temperature does not rush to 50°C, react at 40°C for 3h after the drop is complete, and end the reaction. Wash with water, remove impurity by suction filtration, filtrate layering, keep organic layer, obtain crude product after vacuum distillation reclaims organic solvent, crude product recrystallizes with 30L methanol, obtains 6.2kg white crystal biscarbodiimide compound (I -3), yield 89.33%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com