System and method for preparing high-purity lithium fluoride
A high-purity lithium fluoride and reaction system technology, which is applied in the field of preparation of high-purity lithium fluoride, can solve the problems of high corrosion resistance of equipment and excessive heavy metals in products, and achieve high reliability, improved service life and separation effect Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
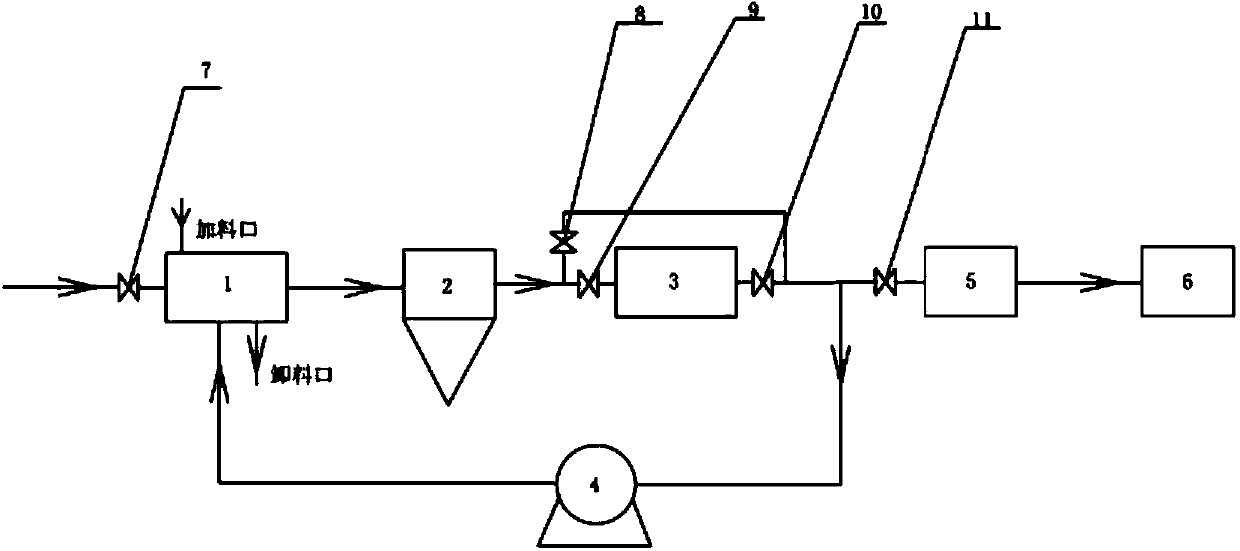
[0032] The preparation process of lithium fluoride uses the reaction of gaseous elemental fluorine gas and solid lithium carbonate to synthesize solid lithium fluoride. The reaction equation is as follows: Li 2 CO 3 +F 2 →2LiF+CO 2 +1 / 2O 2
[0033] The process steps are as follows:
[0034] The gas-solid reactor is pre-filled with solid lithium carbonate and heated, the temperature is less than 400°C, and the fluorine gas enters the reaction system. The reaction system is composed of a circulating fan, a gas-solid reactor, a cyclone separator and a combustion aid, and the pressure of the gas-solid reactor and the reaction system is controlled to be less than 0.3 MPa; the combustion aid is not incorporated into the system when the reaction starts, and when the mixed gas in the circulation system reaches When the pressure is constant, turn on the burner, and the temperature of the burner is 400°C, which is incorporated into the circulation system; when the pressure is const...
Embodiment 1
[0038] First add 10 kg of lithium carbonate in advance to the horizontal gas-solid reactor, and then vacuumize it. Turn on the heater of the gas-solid reactor, and when the temperature is lower than 400°C, open the inlet shut-off valve and slowly introduce fluorine gas to control the system pressure of the gas-solid reactor, cyclone separator and circulating fan to less than 0.06MPa. When not increasing, the system incorporates the burner, and the working temperature of the burner is 400°C. The system continues to circulate, and the first cycle is completed when the pressure does not increase; the system is vacuumed, and then the cycle is continued again, a total of 13 cycles, and the product in the gas-solid reactor is sampled and analyzed for a purity of 99.95%.
Embodiment 2
[0040] First add 8 kg of lithium carbonate to the gas-solid reactor in advance, and then vacuumize it. Turn on the heater of the reaction device, and slowly introduce fluorine gas when the temperature is lower than 350°C, and control the system pressure of the reaction device, cyclone separator and fan to be less than 0.1MPa. When the pressure does not increase, the system is incorporated into the burner , The working temperature of the burner is 350°C. The system continues to circulate, and the first cycle is completed when the pressure does not increase; the system is evacuated, and then continues to cycle again, a total of 16 cycles. The product in the gas-solid reactor device is sampled and analyzed for a product purity of 99.99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com