Preparation method of glycerol monostearate p-hydroxybenzoate
A technology of p-hydroxybenzoic acid and sodium hydroxybenzoate, which is applied in the field of preparation of monoglyceride p-hydroxybenzoate, can solve the problems that glycidol raw materials are difficult to obtain, difficult to obtain, and high price of lipase, and achieve good industrial application prospects and operation Simple and convenient, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
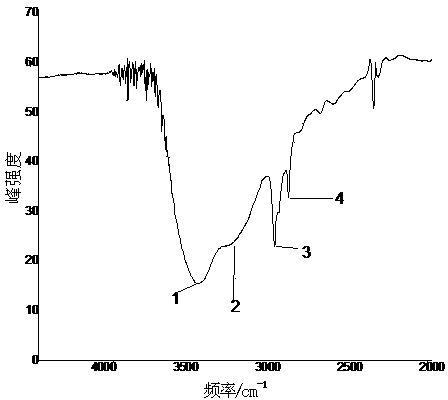
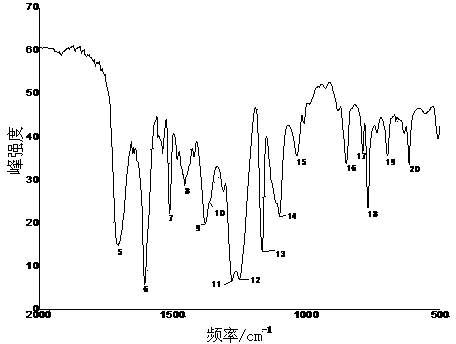
Image
Examples
Embodiment 1
[0021] ①Weigh 2g of sodium p-hydroxybenzoate, 0.03g of tetrabutylammonium bromide, 2ml of epichlorohydrin and 4.2ml of xylene into a sealed stainless steel reactor with magnetic stirring, and react at 115°C for 110 minutes and then The reactor is cooled and opened, and the liquid phase (including unreacted chlorocyclopropane and a large amount of solvent) is poured into a fixed waste liquid bottle, which is used for distilling and recovering the solvent after the reaction is completed. The solid phase was separated from the rotor and transferred to a 50 ml small beaker for later use.
[0022] ② Measure 20ml of 0.02mol / L dilute sulfuric acid solution, pour it into a small beaker containing the intermediate product, and stir for 50 minutes under heating at 80°C. After the reaction, rinse the solid phase with a large amount of deionized water to make the oily and The viscous solid phase is cooled and hardened, and the residual impurities (acid, sodium chloride, catalyst, etc.) ar...
Embodiment 2
[0024] ① Weigh 2g of sodium p-hydroxybenzoate, 0.04g of tetrabutylammonium bromide, 3ml of epichlorohydrin and 5.6ml of xylene into a sealed stainless steel reactor with magnetic stirring, and react at 115°C for 110 minutes The reactor is cooled and opened, and the liquid phase (including unreacted chlorocyclopropane and a large amount of solvent) is poured into a fixed waste liquid bottle, which is used for distilling and recovering the solvent after the reaction is completed. The solid phase was separated from the rotor and transferred to a 50 ml small beaker for later use.
[0025] ② Measure 20ml of 0.03mol / L dilute sulfuric acid solution, pour it into a small beaker containing the intermediate product, and stir for 50 minutes under heating at 80°C. After the reaction, wash the solid phase with a large amount of deionized water to make the oily and The viscous solid phase is cooled and hardened, and the residual impurities (acid, sodium chloride, catalyst, etc.) are washed ...
Embodiment 3
[0027] ① Weigh 2g of sodium p-hydroxybenzoate, 0.05g of tetrabutylammonium bromide, 3.5ml of epichlorohydrin and 7.0ml of xylene into a sealed stainless steel reactor with magnetic stirring, and react at 115°C for 110 minutes Cool and open the reactor, pour the liquid phase (including unreacted chlorocyclopropane and a large amount of solvent) into a fixed waste liquid bottle, and reserve it for distillation and recovery of the solvent after the reaction. The solid phase was separated from the rotor and transferred to a 50 ml small beaker for later use.
[0028] ② Measure 20ml of 0.04mol / L dilute sulfuric acid solution, pour it into a small beaker containing the intermediate product, and stir for 50 minutes under heating at 80°C. After the reaction, rinse the solid phase with a large amount of deionized water to make the oily and The viscous solid phase is cooled and hardened, and the residual impurities (acid, sodium chloride, catalyst, etc.) are washed away, and the obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com