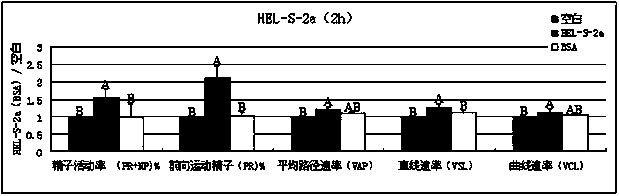
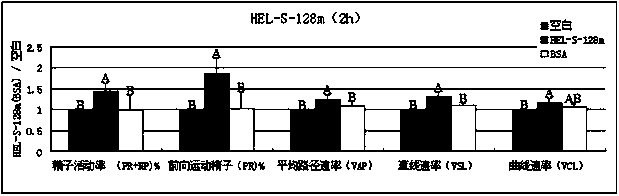
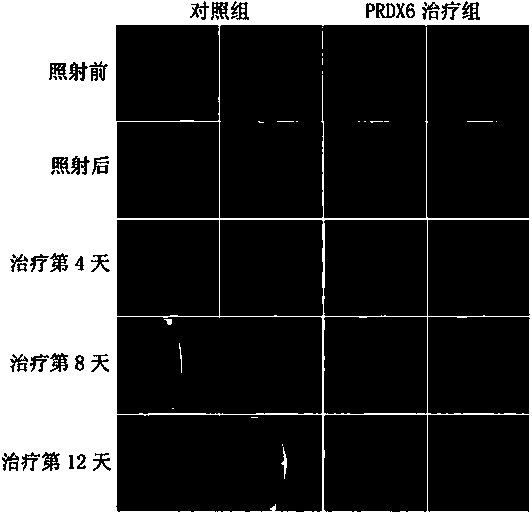
New applications of PRDX2 and/or PRDX6
A use, protein technology, applied in the field of biotechnology and medicine, can solve few problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Example 1 Construction of pET32b (+) / PRDX6 and pET32b (+) / PRDX2 expression vectors
[0147] 1.1 Construction of pET32b(+) / PRDX6 expression vector
[0148] A pair of specific primers were designed and synthesized according to the gene sequence encoding PRDX6 mature protein,
[0149] Upstream primer: 5'-tatccatatgcccggaggtctgcttc-3' (shown in SEQ ID NO:5);
[0150] Downstream primer: 5'-ttactcgagaggctggggtgtgtagcg-3' (as shown in SEQ ID NO: 6);
[0151] The enzyme cutting sites are NdeI and XhoI respectively,
[0152] Human epididymis mRNA (or human epididymis cDNA library prepared in laboratory) extracted by conventional methods was used as template for RT-PCR amplification. see results figure 1 , a 688bp amplified fragment consistent with the theoretical value was detected.
[0153] Separate the amplified fragment (recover the target fragment from the agarose gel with a gel extraction kit), and perform double enzyme digestion with NdeI and XhoI. Then, the double-d...
Embodiment 2
[0158] Expression of embodiment 2PRDX6 protein and PRDX2 protein
[0159] 2.1 Expression of PRDX6 protein
[0160] The plasmid pET32b(+) / PRDX6 was transformed into Escherichia coli Origami B(DE3) (purchased from Novagen), and positive clones were inoculated in LB medium containing 100ug / ml ampicillin, and cultured overnight at 37 degrees in a shaker.
[0161] The next day, transfer to LB medium containing 100ug / ml ampicillin at a ratio of 1:100, culture on a 37-degree shaker until the bacterial density OD600=0.6-0.8, then add IPTG to a final concentration of 0.4mM to induce the expression of the target protein PRDX6 , After 3-4h, the cells were collected by centrifugation.
[0162] 2.2 Expression of PRDX2 protein
[0163] Complete the expression of PRDX2 protein, the expression method is the same as 2.1, the difference is that PRDX2 protein is used instead of PRDX6 protein.
Embodiment 3
[0164] Purification of embodiment 3PRDX6 protein and PRDX2 protein
[0165] 3.1 Purification of PRDX6 protein
[0166] The bacteria collected by centrifugation were resuspended in buffer A (20mM phosphate buffer, 150mM sodium chloride, pH7.2), ultrasonically disrupted in an ice bath, centrifuged at 20,000g at low temperature (4°C) for 15min, and the supernatant was taken as the purified sample.
[0167] After equilibrating the nickel affinity chromatography column with buffer A, the sample was applied to the column, and then the column was washed with buffer A containing 50 mM imidazole to remove impurities (peak 1) (see figure 2 ), the target protein was eluted with buffer A containing 300mM imidazole (peak 2) (see figure 2 ).
[0168] Finally, the eluted protein was replaced with buffer A by G-25 desalting column to remove imidazole, and detected by SDS-PAGE electrophoresis.
[0169] After analysis, the PRDX6 protein with a purity of more than 95% and a molecular weigh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com