Application of 3,4-Dihydroxyphenylacetic acid in preparing antitumor drug
A technology for high altitude catechin and uses, which is applied in the field of antitumor drugs and can solve the problems of unclear toxic components and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Effect of high protocatechuic acid on tumor cell and normal cell proliferation
[0035] Experimental scheme: using thiazolium blue (3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide, MTT) experiment, plateau catechin (DO) (dose gradient of 10mg / ml, 1mg / ml, 100μg / ml, 10μg / ml, 1μg / ml, 100ng / ml) to treat lung cancer cell A549, lung cancer cell LLC, Wilms tumor cell G401, nasopharyngeal cancer cell CNE2Z and laryngeal cancer cell Hep2, rhabdomyosarcoma cell A204, breast cancer cell MDA231, breast cancer cell T47D, breast cancer cell MCF7, pancreatic cancer cell PANC1, neuroblastoma cell SY5Y and cervical cancer cell Hela for 24 hours, MTT staining for 4 hours, DMSO (DMSO) was dissolved, and the absorbance value was measured at 570nm by a microplate reader. With the compound gradient as the abscissa and the cell viability as the ordinate, draw a standard curve and calculate the half inhibitory concentration IC 50 , see Table 1 for the specific results.
...
Embodiment 2
[0039] Example 2 In vitro anti-tumor cell migration effect of plateau catechin
[0040] Experimental program:
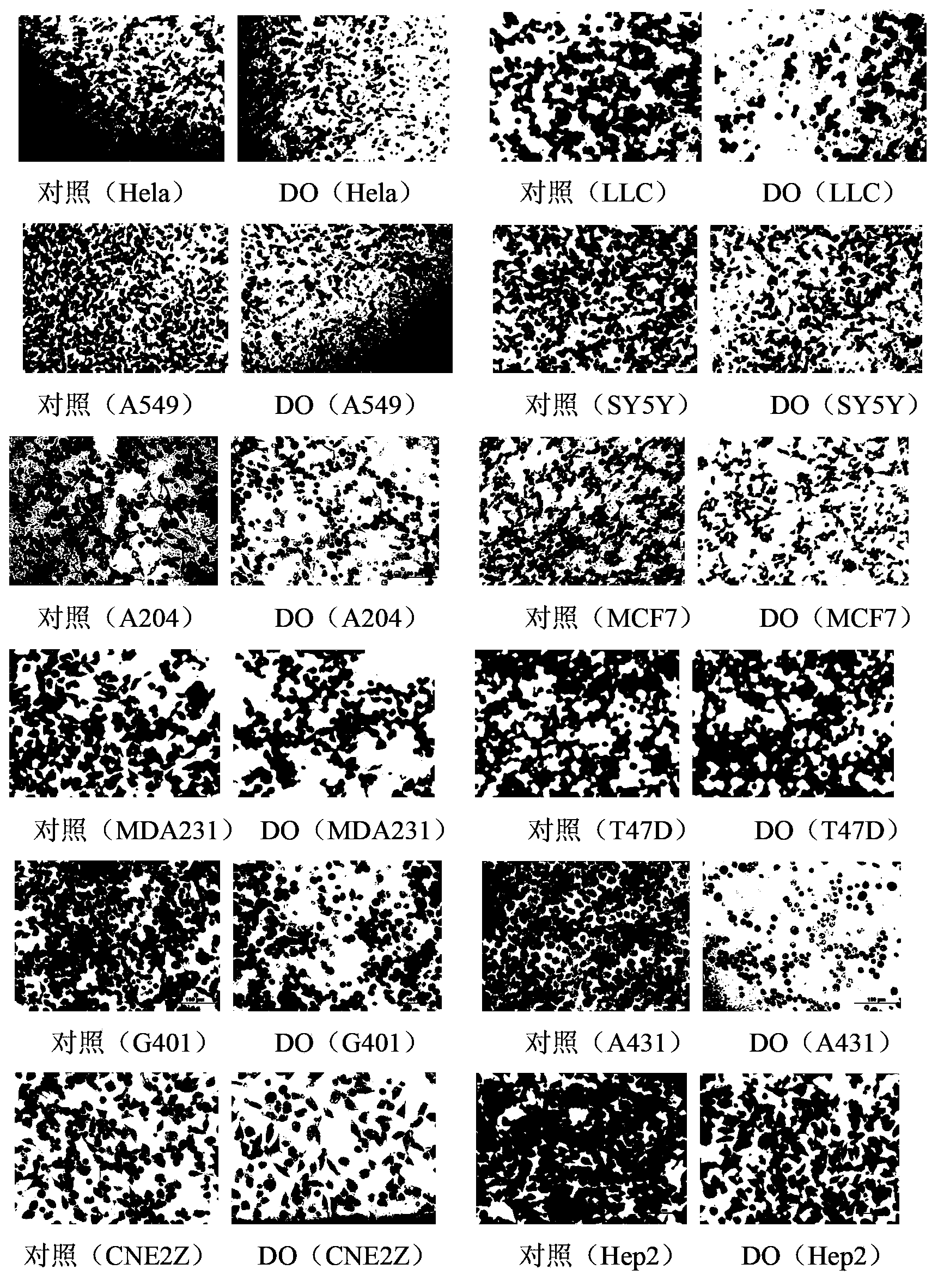
[0041] 1) Using cell migration assay (Transwell) experiment, 20 μg / ml high protocatechuic acid (DO) (safe dose) to treat lung cancer cells LLC, lung cancer cells (A549), cervical cancer cells Hela, neuroblastoma cells SY5Y, breast cancer cells Cell MCF7, breast cancer cell MDA231, breast cancer cell T47D, rhabdomyosarcoma cell A204, Wilms tumor cell G401, skin squamous cell carcinoma cell A431, nasopharyngeal carcinoma cell CNE2Z and laryngeal carcinoma cell Hep224 hours later, anhydrous ice methanol fixation 20 Minutes, then stained with crystal violet for 15 minutes, and photographed with a 100-fold light microscope to detect the number of cell migration.
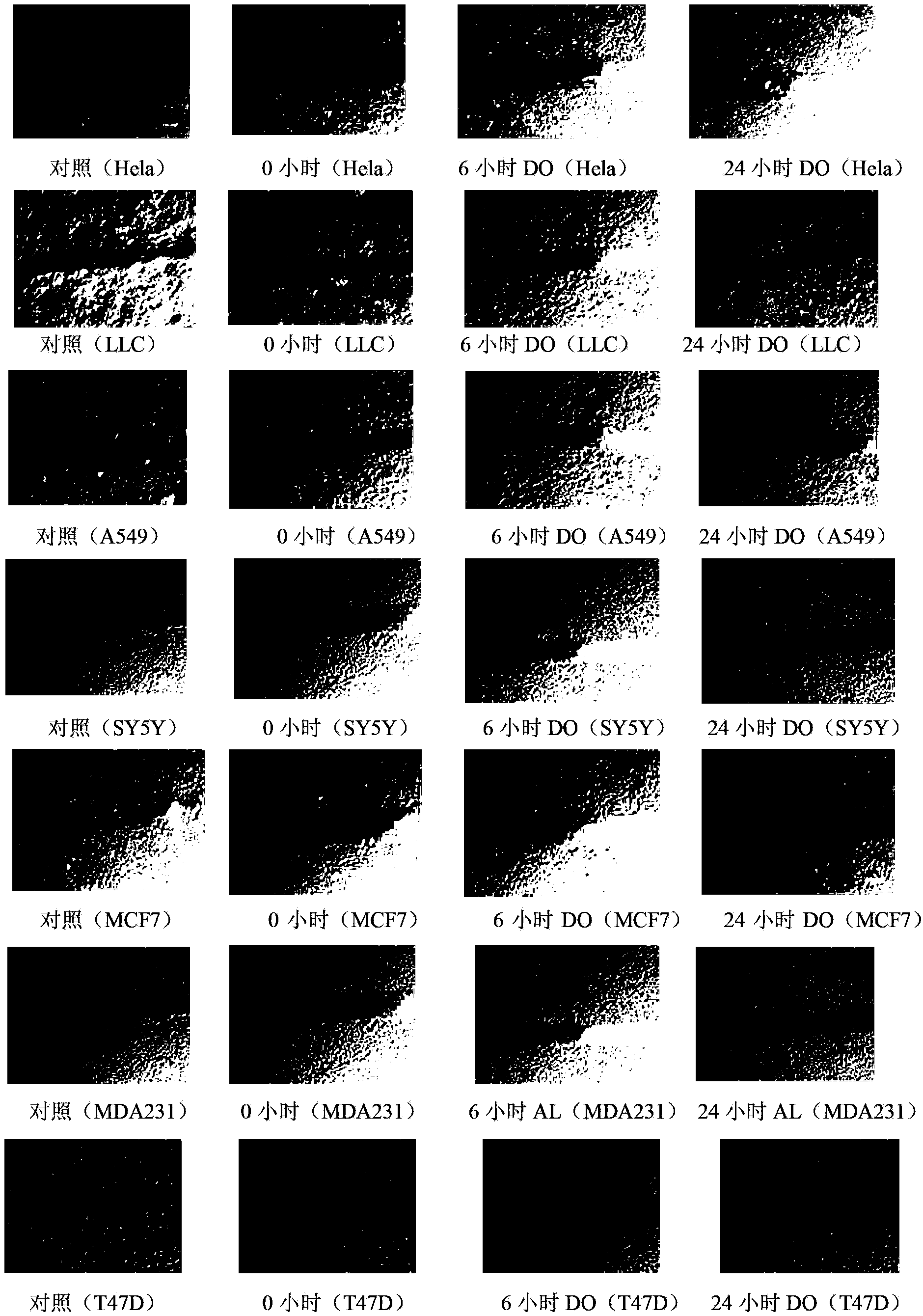
[0042] 2) Using cell migration assay (Scratch Analysis) experiment, 20μg / ml high protocatechuic acid (DO) (safe dose) to treat lung cancer cells (LLC), lung cancer cells (A549), cervical cancer cells (Hela), ne...
Embodiment 3
[0047] Example 3 Effect of high protocatechuic acid on tumor cell apoptosis
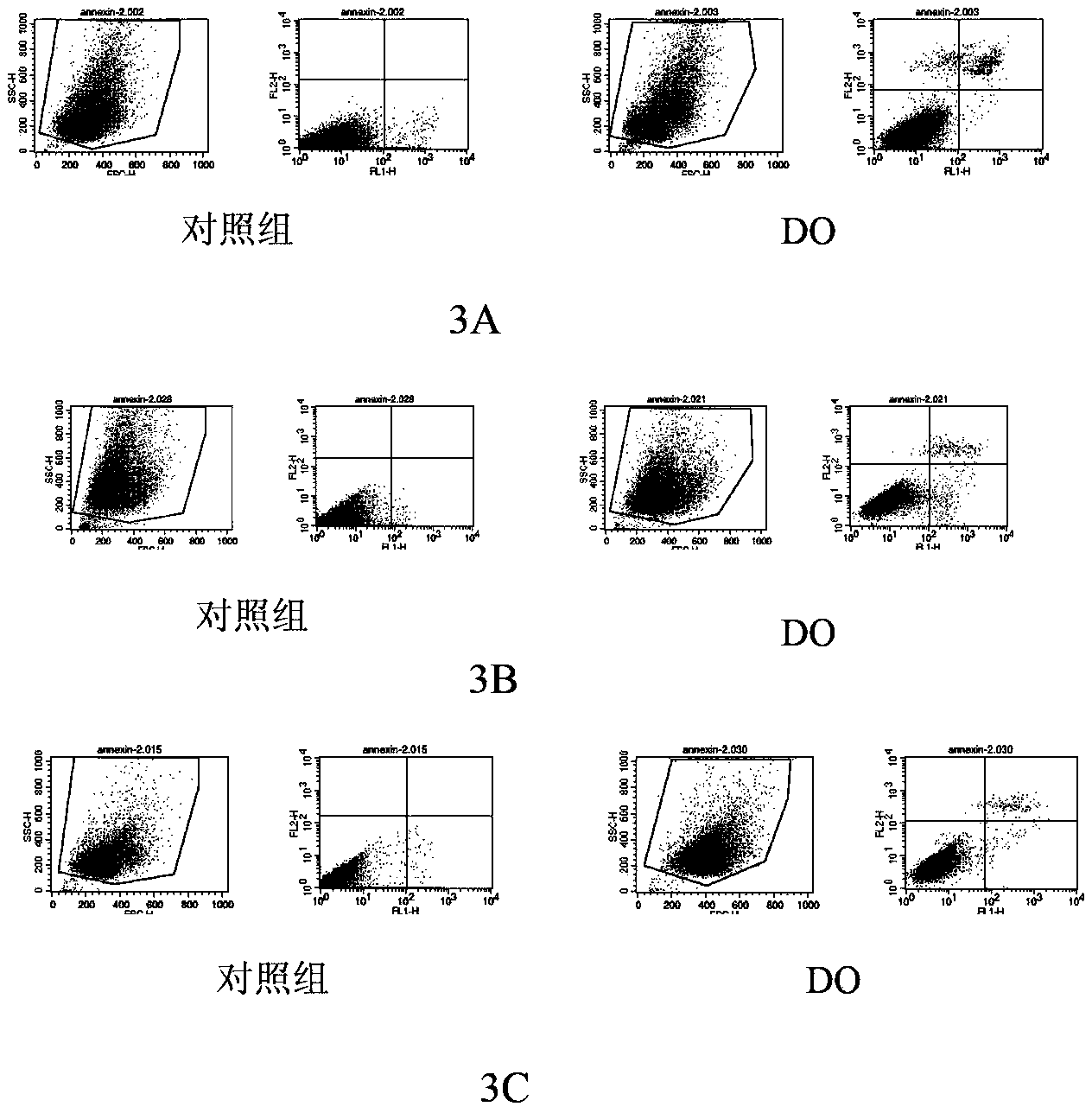
[0048] Experimental protocol: Apoptotic flow cytometry assay was used. Human lung cancer cells (A549), Wilms tumor cells (G401) and laryngeal carcinoma cells (Hep2) were treated with 10 μM high catechin (DO) for 24 hours, and collected by centrifugation Cells (1500rpm, 5min), resuspended in PBS and centrifuged for 2 times, then stained with Annexin-V FITC and PI for 15min respectively, the percentage of apoptotic cells, the sum of the percentages of early apoptotic cells and late apoptotic cells were detected by flow cytometry and the total ratio of apoptosis.
[0049] Experimental results: Higher catechin (DO) can significantly promote the apoptosis of A549, G401 and Hep2 cells, suggesting that higher level catechin (DO) may play an anti-tumor effect by mediating the apoptosis of tumor cells. For specific results, see image 3 A-3C.
[0050] The above in vitro anti-tumor experiment results show t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com