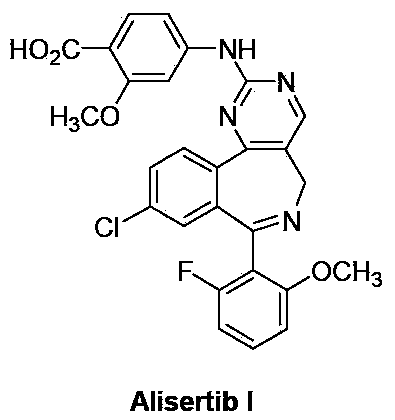
Method for preparing Alisertib
A technology of arisetide and methoxyphenyl, which is applied in the field of preparation of arisetide, can solve problems such as low reaction yield, difficult purification, and increased difficulty in industrialization, and achieves easy-to-obtain raw materials, simple process, Conducive to the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] -20°C and dry nitrogen atmosphere, add sodium hydride (0.5g, 20mmol) and acetonitrile 5mL to the reaction flask, stir for 30 minutes and then slowly rise to 0°C, dropwise add 3-[(2-fluoro-6-methoxy Base) phenyl]-5-chlorophthalide (II) (2.92g, 10mmol) in acetonitrile 20mL solution, warmed up to room temperature, stirred for 24 hours, TLC detected the end of the reaction. Dichloromethane and water were added to quench the reaction, the organic phase was separated, the aqueous phase was adjusted to pH 7.5-8.5 with hydrochloric acid, and extracted three times with dichloromethane. Dry over anhydrous sodium sulfate, distill under reduced pressure to recover the solvent, and recrystallize the residue with dichloromethane and petroleum ether to obtain off-white solid 1,3-dihydro-1-hydroxy-3-[(2-fluoro-6-methanol) Oxy)phenyl]-5-chloro-isobenzofuran-1-acetonitrile (III) 2.10 g, yield 63.1%.
Embodiment 2
[0027] At room temperature, add 1,3-dihydro-1-hydroxyl-3-[(2-fluoro-6-methoxy)phenyl]-5-chloro-isobenzofuran-1-acetonitrile ( III) (1.67g, 5mmol) and N,N-dimethylformamide dimethyl acetal (DMF-DMA) 15mL, heated up to 60-65°C, stirred for 18 hours, and TLC detected the end of the reaction. Unreacted N,N-dimethylformamide dimethyl acetal was removed under reduced pressure. After cooling down to room temperature, the reaction was quenched with water, extracted 3 times with dichloromethane, and dried. The solvent was recovered under reduced pressure to give crude oil 3-{[1-(4-chlorophenyl)-1'-(2-fluoro-6-methoxyphenyl)methanol]-2-yl}-2-[ 2-(Dimethylamino)methylene]-3-oxapropionitrile (IV) 1.75 g, yield 90.2%.
Embodiment 3
[0029] At room temperature, add 3-{[1-(4-chlorophenyl)-1'-(2-fluoro-6-methoxyphenyl)methanol]-2-yl}-2-[2 -(dimethylamino)methylene]-3-oxapropionitrile (IV) (1.94g, 5mmol), 4-guanidine-2-methoxybenzoic acid hydrochloride (VI) (1.27g, 6.25 mmol), potassium tert-butoxide (1.4g, 12.5mmol) and 50mL of methanol, the temperature was raised to reflux, stirred and reacted for 18 hours, and the reaction was detected by TLC. The reaction was quenched with water, adjusted to pH 1-2 with hydrochloric acid, and extracted 3 times with ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate, the solvent was recovered under reduced pressure, and the residue was recrystallized from ethyl acetate and n-hexane to obtain off-white solid 4-{[4-[1-(4-chlorophenyl)-1' -(2-fluoro-6-methoxyphenyl)methanol]-5-cyanopyrimidin-2-yl]amino}-2-methoxybenzoic acid (V) 2.08g, yield 77.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com