Naphthalene-containing liquid crystal epoxy resin compound, its preparation method and composition
A technology for epoxy resins and compounds, applied in chemical instruments and methods, liquid crystal materials, organic chemistry, etc., can solve problems such as side reactions, yield reduction, etc., to improve yield, improve heat resistance, and improve product purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
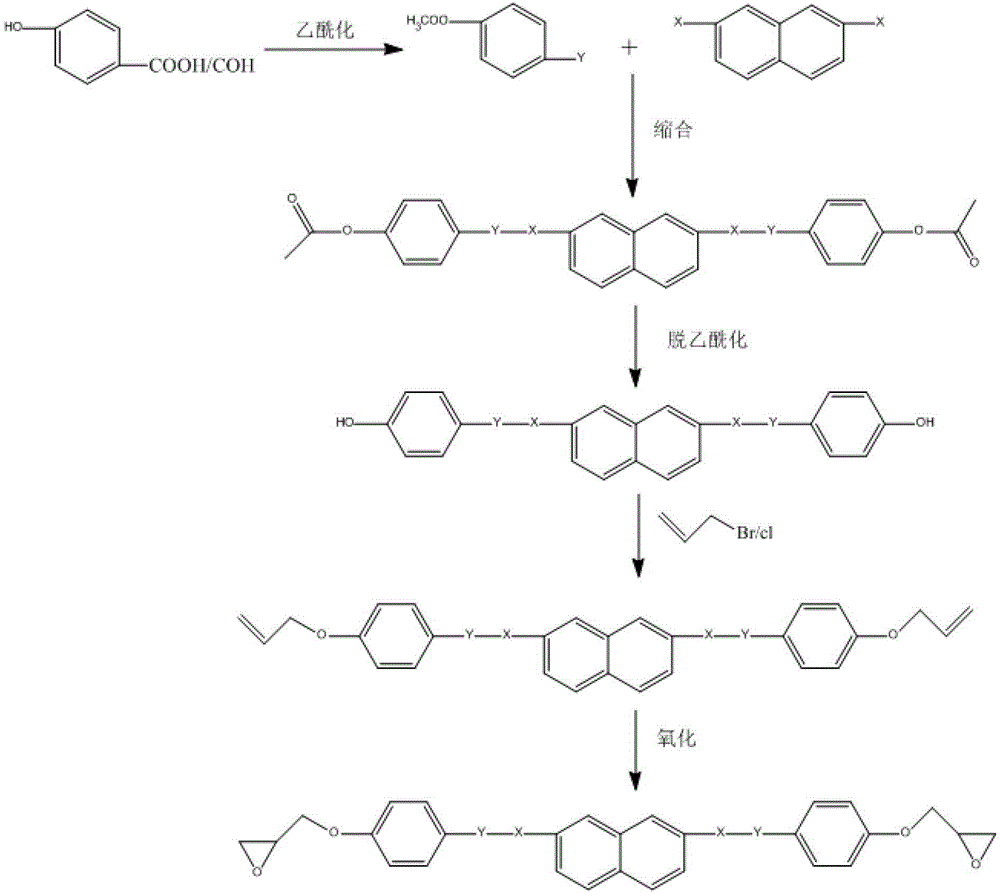
[0027] In the preparation method of the present invention, the double bond oxidation method is adopted to form the skeleton structure and then the alkenyl is introduced, and then the alkenyl is epoxidized with a peroxidant, thereby greatly reducing the by-products in the reaction and improving the purity and reaction of the product. yield, and also simplifies the purification operation. The product can be isolated and purified, for example, by simple column chromatography. Moreover, due to the double bond oxidation method, the reaction can be carried out in a wider time range and a larger temperature range, and the reaction control is more convenient.
[0028] Specifically, the catalyst used in step S1 can be 18-crown-6-ether, 15-crown-5-ether, dibenzo-18-crown-6-ether, or any mixture thereof; the catalyst and the The molar ratio of the phenolic compounds can be (0.005-0.5): 1; an appropriate amount of potassium carbonate, sodium carbonate, sodium hydroxide, sodium bicarbonat...
Embodiment 1
[0040] 0.05 mol of p-hydroxybenzoic acid was acylated under the catalysis of 10 parts of acetic anhydride, and reacted at 80° C. for 5 hours to obtain acetoxybenzoic acid.
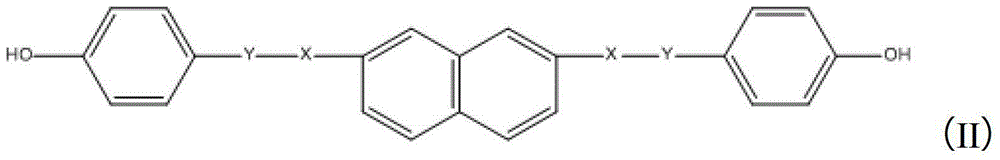
[0041] 0.03mol of acetoxybenzoic acid and 0.013mol of 2,7-dihydroxynaphthalene were dissolved in 80ml of acetone, with pyridine and thionyl chloride as catalysts, reacted at 0°C for 12 hours, and separated by chromatography to obtain 4,4'-bis( 4-acetoxybenzyloxy)-2,7-naphthalene.
[0042] 0.011mol 4,4'-bis(4-acetoxybenzyloxy)-2,7-naphthalene was dissolved in 60ml acetone, under the catalysis of ammonia water, reacted at room temperature for 12 hours to obtain 4,4'-bis(4-hydroxy Benzyloxy)-2,7-naphthalene.
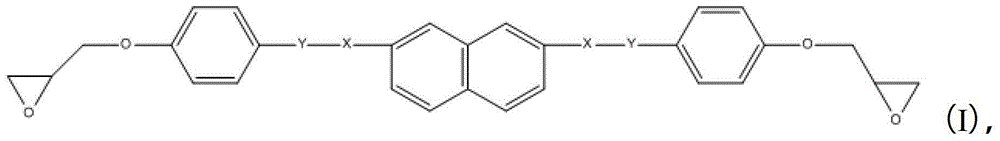
[0043]Dissolve 0.01mol 4,4′-bis(4-hydroxybenzyloxy)-2,7-naphthalene and 0.011mol propylene bromide in acetone, add appropriate amount of potassium carbonate and 18-crown-6-ether, and react at 65°C for 24 product obtained in hours. After purification by column chromatography, it was dissolved in dichl...
Embodiment 2
[0047] 0.05 mol of p-hydroxybenzoic acid was acylated under the catalysis of 12 parts of acetyl chloride, and reacted at 90° C. for 5 hours to obtain acetoxybenzoic acid.
[0048] 0.03mol of acetoxybenzoic acid and 0.013mol of 2,7-dihydroxynaphthalene were dissolved in 80ml of acetone, with pyridine and thionyl chloride as catalysts, reacted at -3°C for 12 hours, and separated by chromatography column to obtain 4,4'-bis (4-Acetoxybenzyloxy)-2,7-naphthalene.
[0049] 0.011mol 4,4'-bis(4-iminobenzyloxy)-2,7-naphthalene was dissolved in 70ml tetrahydrofuran, under the catalysis of ammonia water, reacted at room temperature for 12 hours to obtain 4,4'-bis(4-hydroxybenzene imino)-2,7-naphthalene.
[0050] Dissolve 0.01mol 4,4′-bis(4-hydroxybenzyloxy)-2,7-naphthalene and 0.011mol chloropropene in tetrahydrofuran, add appropriate amount of potassium bicarbonate and 18-crown-6-ether, and react at 80°C The product obtained in 24 hours. After purification by column chromatography, it...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com