Method for preparing nano ferroferric oxide
A ferric oxide and nanotechnology, applied in the field of nanomaterials, can solve the problems of difficult control of process parameters, complex chemical process, difficult mass production, etc., and achieve the effect of realizing mass production, low equipment requirements, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Get 20 grams of ferrous oxalate and add it in the ceramic boat, the material layer thickness is 2.5cm. The ceramic boat that ferrous oxalate is housed is placed on the middle position of heating furnace heating band, then feeds high-purity argon gas into heating furnace according to the speed of 5ml / min.
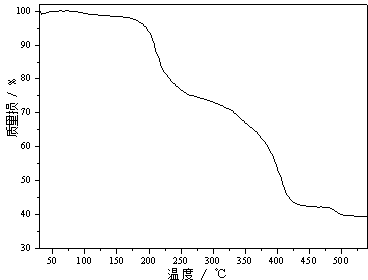
[0023] After the air in the heating furnace is completely replaced by high-purity argon, continue to introduce high-purity argon, control the heating furnace to raise the temperature to 510°C at a rate of 18°C / min, and then keep it at 510°C for 18 minutes to carry out the decomposition reaction.
[0024] After the decomposition reaction, the heating furnace was powered off, and the high-purity argon gas continued to flow in. After the temperature of the heating furnace dropped to 40°C, the high-purity argon flow was stopped, and the material 1 was obtained by cooling to room temperature (18-25°C). The mass of material 1 was 11.318 g, and the yield was 56.59%.
[0025...
Embodiment 2
[0028] Get 20 grams of ferrous oxalate and add it in the ceramic boat, the material layer thickness is 2cm. The ceramic boat that ferrous oxalate is housed is placed on the middle position of the heating furnace heating band, then feeds high-purity argon into the heating furnace according to the speed of 4ml / min.
[0029] After the air in the heating furnace is completely replaced by high-purity argon, continue to feed high-purity argon, control the heating furnace to heat up to 500°C at a rate of 15°C / min, and then keep it at 500°C for 20 minutes to carry out the decomposition reaction.
[0030] After the decomposition reaction, the heating furnace was powered off, and the high-purity argon was continued to flow in. After the temperature of the heating furnace dropped to 30°C, the high-purity argon flow was stopped, and the material 2 was obtained by cooling to room temperature (18-25°C). The mass of material 2 was 11.323 g, and the yield was 56.62%.
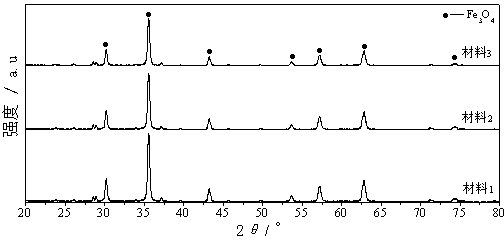
[0031] Through the X-r...
Embodiment 3
[0034] Get 20 grams of ferrous oxalate and add it in the ceramic boat, the material layer thickness is 3cm. The ceramic boat that ferrous oxalate is housed is placed on the middle position of heating furnace heating band, then feeds high-purity argon gas into heating furnace according to the speed of 3ml / min.
[0035] After the air in the heating furnace is completely replaced by high-purity argon, continue to feed high-purity argon, control the heating furnace to raise the temperature to 520°C at a rate of 20°C / min, and then keep it at 520°C for 15 minutes to carry out the decomposition reaction.
[0036] After the reaction, the heating furnace was powered off, and the high-purity argon gas continued to flow in. After the temperature of the heating furnace dropped to 35°C, the high-purity argon flow was stopped, and the material 3 was obtained by cooling to room temperature (18-25°C). The mass of material 3 was 11.314 g, and the yield was 56.57%.
[0037] Through the X-ray d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com