Preparation method of syringopicroside (SYR) poly(lactide-co-glycolide) (PLGA) nanoparticles
A technology of syringoside and -PLGA, which is applied in the field of preparation of syringoside PLGA nanoparticles, can solve the problems of large oral dose of syringoside, affecting the effect of liver disease, poor compliance of patients taking medicine, etc. Good sealing rate and drug loading, and the effect of increasing bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
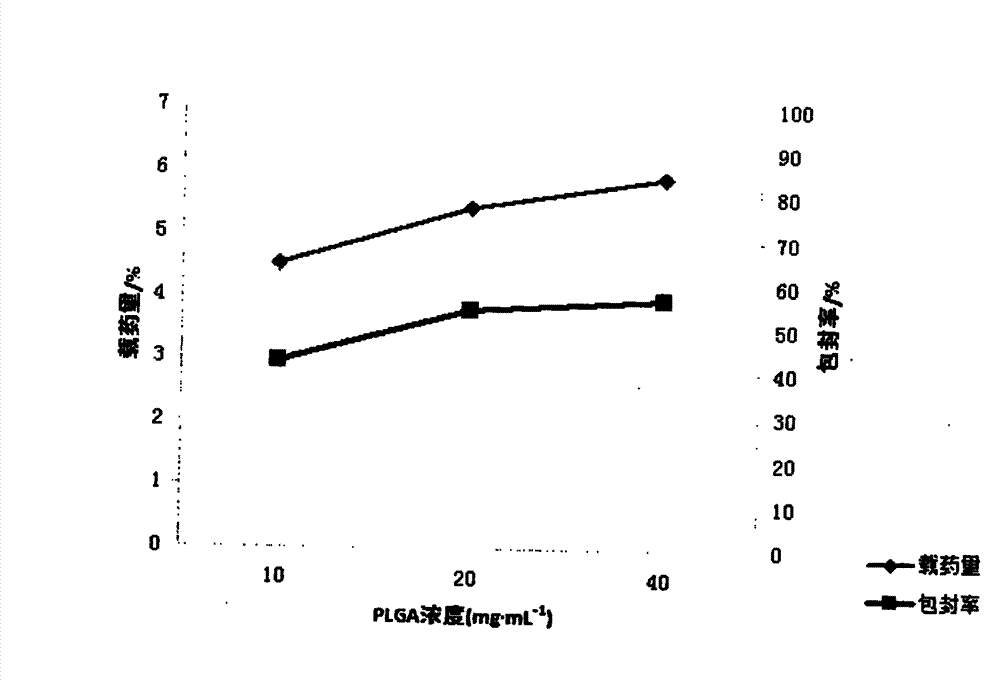
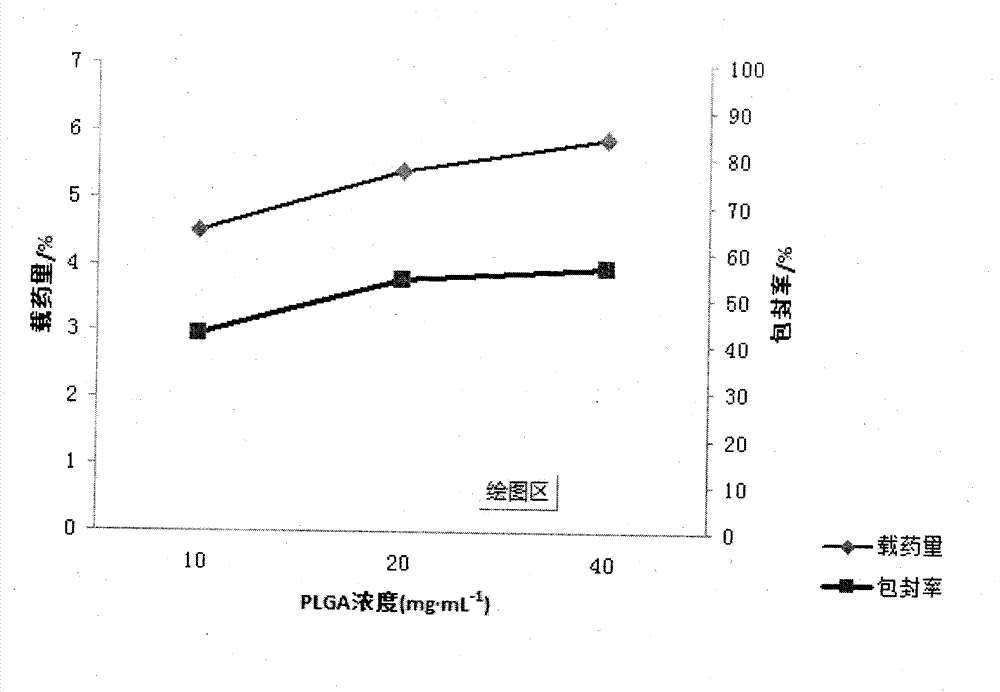
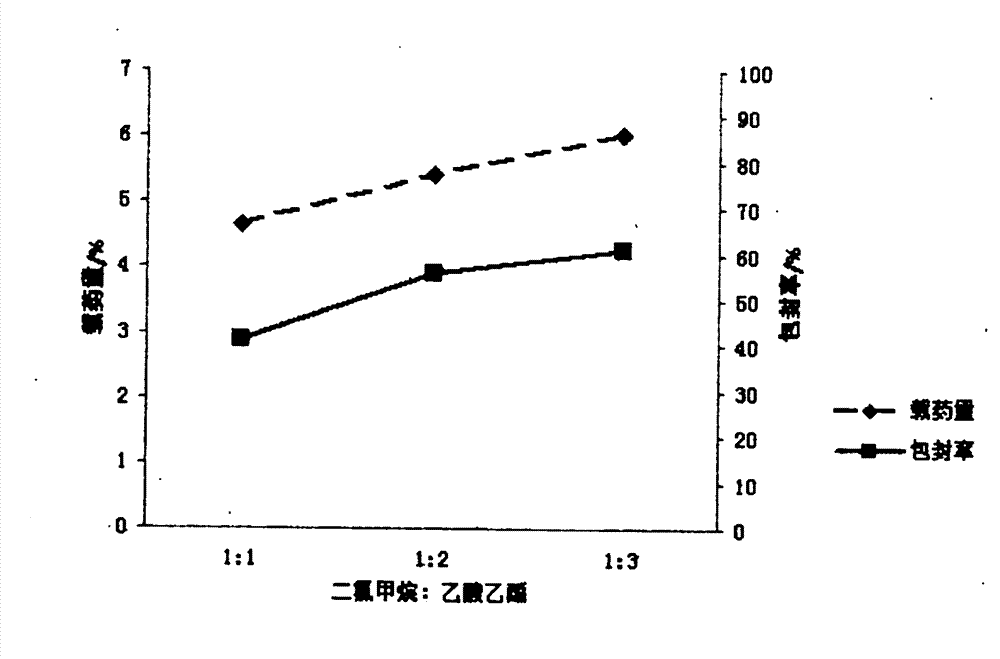
[0022] Embodiment 1 First, in order to achieve the above object, the technical scheme adopted in the present invention is: first, take PLGA (LA:GA=50:50) and join in the mixed solution of dichloromethane and ethyl acetate 1:1, obtain Concentration is the PLGA solution of 15mg / ml, then syringin is dissolved in distilled water, makes the medicine solution of 8mg / ml; Secondly, according to the volume ratio of 1:3, medicine is added in the PLGA solution, ultrasonic emulsification, makes Colostrum, then, inject this colostrum into poloxamer at a mass concentration of 0.5% 188 The aqueous solution forms the primary double emulsion, and then the primary double emulsion is added to 0.5% poloxamer according to the volume of 1:6 times 188 After 1-2 hours of magnetic stirring at 800r / min, the mixed solution of dichloromethane and ethyl acetate was rotated under reduced pressure for 45-60min to volatilize to form a colloidal solution. After freeze-drying, syringin-PLGA Nanoparticles
Embodiment 2
[0023] Embodiment 2 First, in order to achieve the above object, the technical scheme adopted by the present invention is: first, take PLGA (LA:GA=50:50) and join in the mixed solution of dichloromethane and ethyl acetate 1:2, obtain Concentration is the PLGA solution of 20mg / ml, then syringin is dissolved in distilled water, makes the medicine solution of 10mg / ml; Secondly, according to the volume ratio of 1:11, medicine is added in the PLGA solution, ultrasonic emulsification, makes Colostrum, then, inject the colostrum to a mass concentration of 0.5% poloxamer 188 The aqueous solution forms the primary double emulsion, and then the primary double emulsion is added to 1% poloxamer according to the volume of 1:11 times 188 After 1-2 hours of magnetic stirring at 800r / min, the mixed solution of dichloromethane and ethyl acetate was rotated under reduced pressure for 45-60min to volatilize to form a colloidal solution. After freeze-drying, syringin-PLGA Nanoparticles
Embodiment 3
[0024] Embodiment 3 First, in order to achieve the above object, the technical scheme adopted by the present invention is: first, take PLGA (LA:GA=50:50) and join in the mixed solution of dichloromethane and ethyl acetate 1:3, obtain Concentration is the PLGA solution of 18mg / ml, then syringin is dissolved in distilled water, makes the medicine solution of 15mg / ml; Secondly, according to the volume ratio of 1:4, medicine is added in the PLGA solution, ultrasonic emulsification, makes Colostrum, then, inject this colostrum into poloxamer at a mass concentration of 0.5% 188 The aqueous solution forms the primary double emulsion, and then the primary double emulsion is added to 0.75% poloxamer according to the volume of 1:10 times 188 After 1-2 hours of magnetic stirring at 800r / min, the mixed solution of dichloromethane and ethyl acetate was rotated under reduced pressure for 45-60min to volatilize to form a colloidal solution. After freeze-drying, syringin-PLGA Nanoparticles ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com