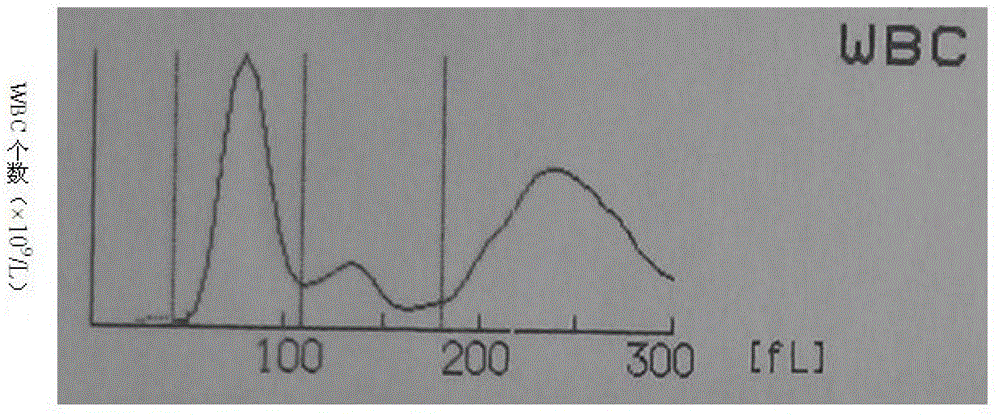
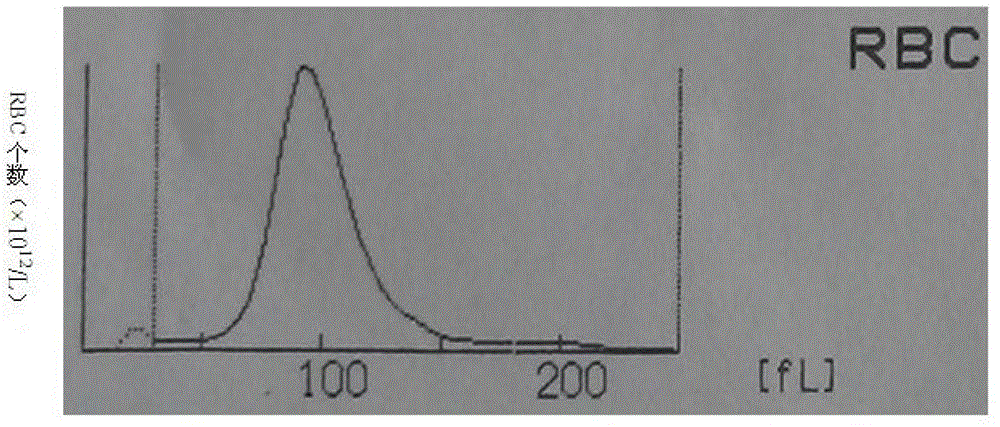
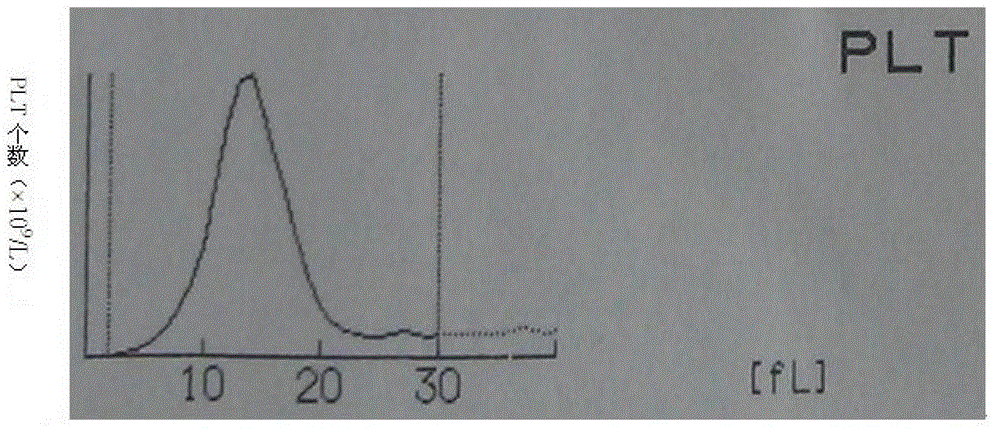
Whole blood quality control material and preparation method thereof
A quality control substance and blood quality technology, applied in the field of clinical testing, can solve the problems of complex extraction process, platelet count interference, high cost, etc., and achieve the effect of simple production process, obvious size difference, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Anticoagulant: In 1L of anticoagulant, glucose is 32.4g, citric acid is 3.85g, sodium dihydrogen phosphate is 4.82g, and the balance is water.
[0035] PBS buffer: In 1L of PBS buffer, 4.5g of sodium chloride, 22.1g of disodium hydrogen phosphate, and 2.1g of sodium dihydrogen phosphate.
[0036] White blood cell fixative: formaldehyde: glutaraldehyde (V:V) = 1:1 mixed solution, the final concentration of formaldehyde and glutaraldehyde is 5%.
[0037] Red blood cell and platelet fixative: in 1L red blood cell and platelet fixative, ATP 2.5g, lactose 75g, formaldehyde 0.85ml, F-680.36g, citric acid 0.5g, trisodium citrate 14.2g, sodium azide 3.0g , and the rest is water.
Embodiment 2
[0039] Anticoagulant: In 1L of anticoagulant, ethylenediaminetetraacetic acid 28.4g, citric acid 1.6g, sodium dihydrogen phosphate 1g, and the balance is water.
[0040] PBS buffer: In 1L of PBS buffer, 4.5g of sodium chloride, 22.1g of disodium hydrogen phosphate, and 2.1g of sodium dihydrogen phosphate.
[0041] White blood cell fixative: paraformaldehyde: F-68 (V:V) = 1:1 mixed solution, the final concentration is 0.05%.
[0042] Red blood cell and platelet fixative: in 1L red blood cell and platelet fixative, adenine 2.5g, glucose 47.5g, paraformaldehyde 0.3g, F-680.2g, citric acid 0.3g, trisodium citrate 10.2g, azide Sodium 0.5g, the rest is water.
Embodiment 3
[0044] Anticoagulant: In 1L of anticoagulant, glucose is 65.4g, trisodium citrate is 9.6g, sodium dihydrogen phosphate is 5.2g, and the balance is water.
[0045] PBS buffer solution: In 1L of PBS buffer solution, 5.5 g of sodium chloride, 20.3 g of disodium hydrogen phosphate, and 3.1 g of sodium dihydrogen phosphate.
[0046] White blood cell fixative: formaldehyde: glutaraldehyde (V:V) = 1:1 mixed solution, the final concentration of formaldehyde and glutaraldehyde is 10%.
[0047] Red blood cell and platelet fixative: in 1L red blood cell and platelet fixative, ATP10g, lactose 110g, formaldehyde 2ml, F-684.0g, disodium hydrogen phosphate 3.0g, sodium dihydrogen phosphate 2.0g, sodium azide 4.0g, the rest for water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com