Nano nickel oxide coated modified boron fuel and preparation methods thereof
A nano-nickel oxide and nano-oxidation technology, which is applied in the direction of explosives, can solve the problems of refractory melting and gasification, low combustion efficiency of boron, high melting point and boiling point, and achieve stable combustion state, high combustion efficiency and good fuel performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
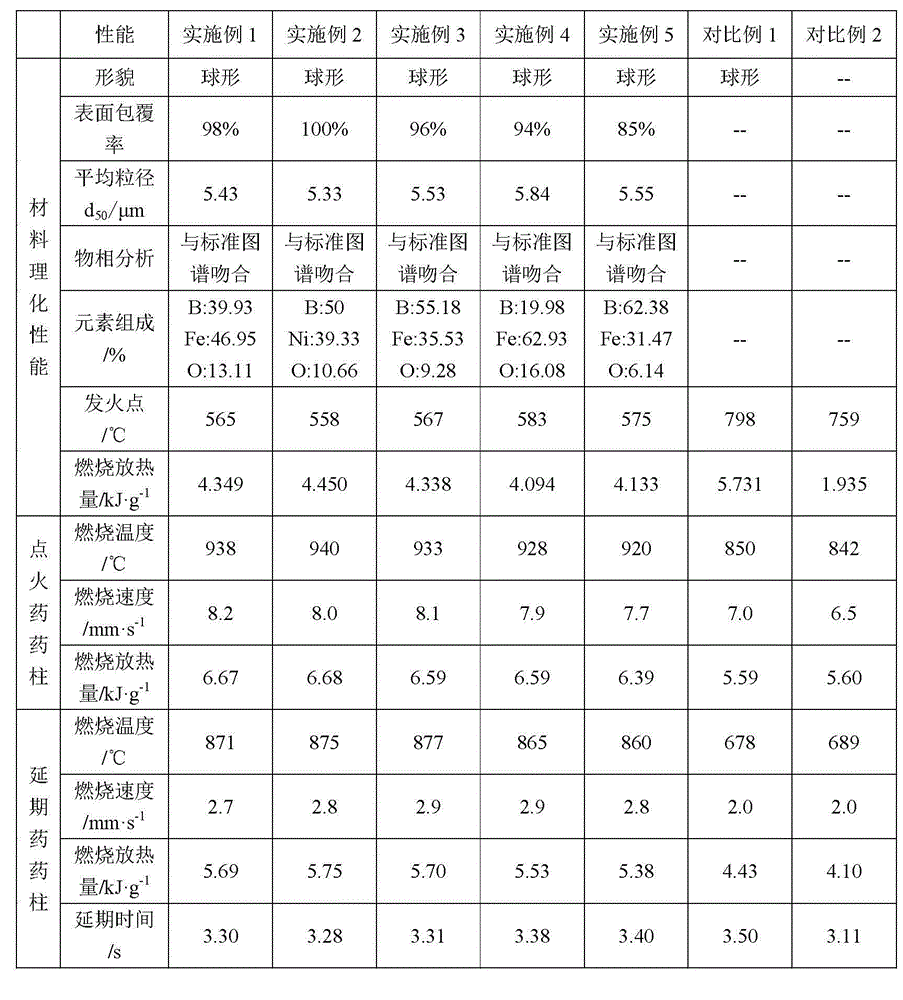
[0062] Example 1 Modified boron fuel prepared by precipitation method
[0063] Taking the target product mass of 1.5g (the mass ratio of boron to nickel oxide is 40:60) as an example, the amount of various raw materials required is: nickel chloride hexahydrate 2.856g, sodium hydroxide 1.28g, superfine boron powder 0.6g (boron powder particle size 4.9μm), 150ml ethanol. Accurately weigh quantitative nickel chloride hexahydrate and sodium hydroxide, and dissolve them in ethanol respectively; under normal temperature and stirring conditions, add ultrafine boron powder to the nickel chloride solution, and stir well to make it evenly dispersed in the solution middle; add sodium hydroxide solution dropwise to the mixed suspension to make it fully react, filter the product, wash with deionized water and ethanol for 3 times respectively, vacuum dry at 80°C for 4h, and calcinate at 400°C for 2h, the obtained product It is the nano-nickel oxide-coated modified boron fuel of this patent...
Embodiment 2
[0065] Example 2 Modified boron fuel prepared by precipitation method
[0066] Taking the target product mass of 1.8g (mass ratio of boron to nickel oxide is 50:50) as an example, the amount of various raw materials required is: 2.856g of nickel chloride hexahydrate, 1.28g of sodium hydroxide, superfine boron powder 0.9g (boron powder particle size 5.07μm), 150ml ethanol. Accurately weigh quantitative nickel chloride hexahydrate and sodium hydroxide, and dissolve them in ethanol respectively; under normal temperature and stirring conditions, add ultrafine boron powder to the nickel chloride solution, and stir well to make it evenly dispersed in the solution middle; add sodium hydroxide solution dropwise to the mixed suspension to make it fully react, filter the product, wash with deionized water and ethanol for 3 times respectively, vacuum dry at 80°C for 4h, and calcinate at 400°C for 2h, the obtained product It is the nano-nickel oxide-coated modified boron fuel of this pat...
Embodiment 3
[0068] Example 3 Modified boron fuel prepared by precipitation method
[0069] Taking the target product mass of 2g (the mass ratio of boron to nickel oxide is 55:45) as an example, the amount of various raw materials required is: 2.856g of nickel chloride hexahydrate, 1.28g of sodium hydroxide, and 1.1g of ultrafine boron powder g (boron powder particle size 5.3 μm), ethanol 150ml. Accurately weigh quantitative nickel chloride hexahydrate and sodium hydroxide, and dissolve them in ethanol respectively; under normal temperature and stirring conditions, add ultrafine boron powder to the nickel chloride solution, and stir well to make it evenly dispersed in the solution middle; add sodium hydroxide solution dropwise to the mixed suspension to make it fully react, filter the product, wash with deionized water and ethanol for 3 times respectively, vacuum dry at 80°C for 4h, and calcinate at 400°C for 2h, the obtained product It is the nano-nickel oxide-coated modified boron fuel ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com