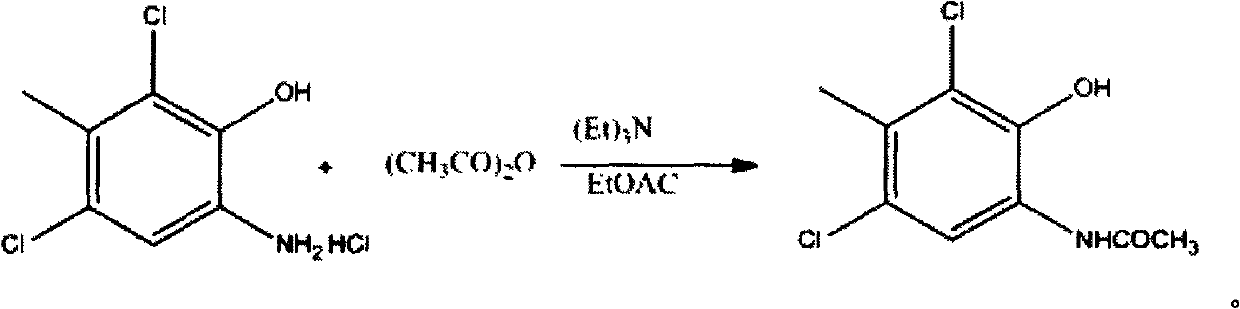Preparation method of indophenol derivative
A technology of derivatives, indophenol, applied in the field of dyeing agent indophenol derivatives and preparation thereof, can solve the problems of large environmental pollution and low yield, and achieve the effects of reducing pollution, improving yield, and being easy to industrialize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put 20g of 1, 150ml of acetonitrile, and 8g (1.1eq.) of sodium acetate into a 250ml three-neck bottle, add 10.3 (1.1eq.) of acetic anhydride dropwise at room temperature, and the white suspension is formed. Slowly add acetic anhydride dropwise, and a large amount of white is precipitated after 20 minutes. The solid was filtered with suction, and the filter cake was washed with 50 ml of water to obtain 21 g of white solid 2,4-dichloro-3-methyl 6-acetaminophen, with a yield of 99%.
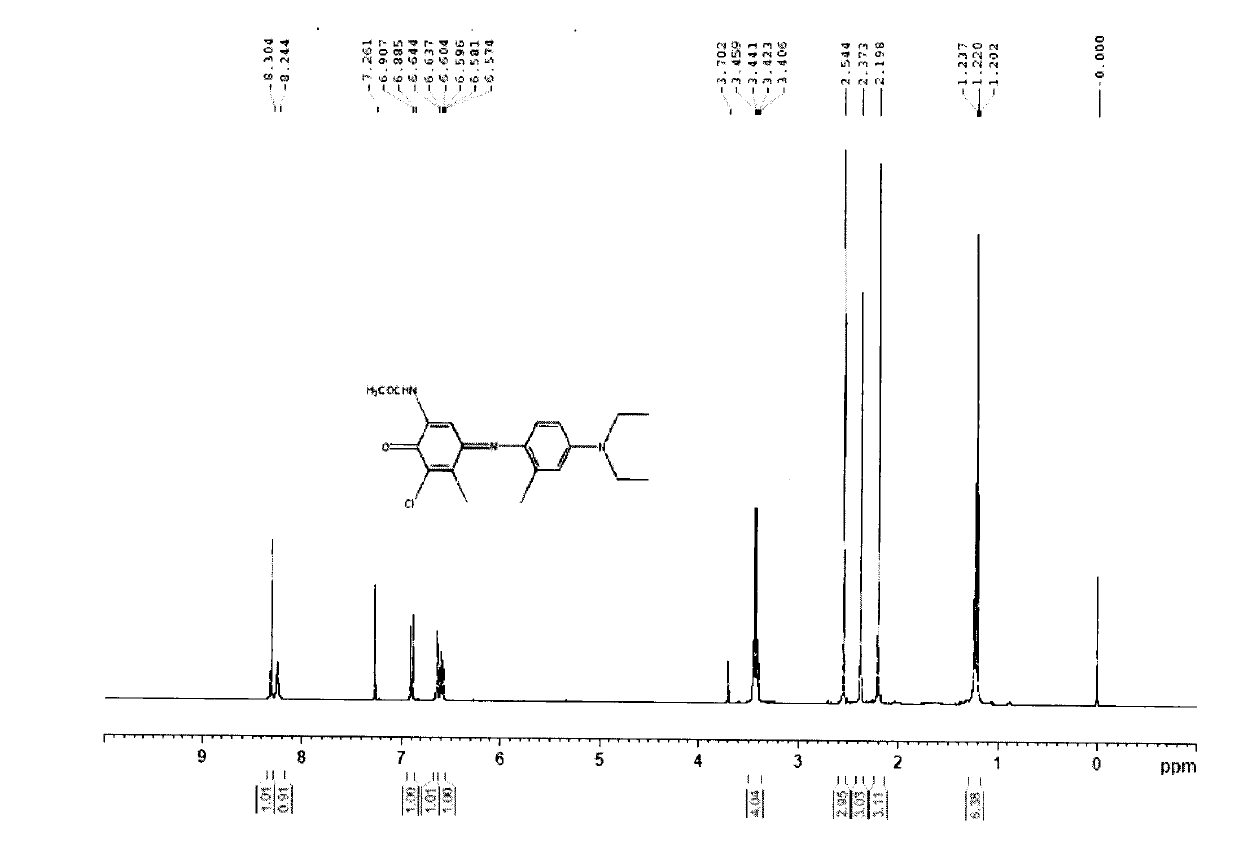
[0021] Have it tested by NMR. 1H NMR (400MHZ, DMSO-d6) δ10.01(1H), δ9.64-9.69(1H), δ7.64(1H), δ2.32-2.38(3H), δ2.07-2.11(3H).
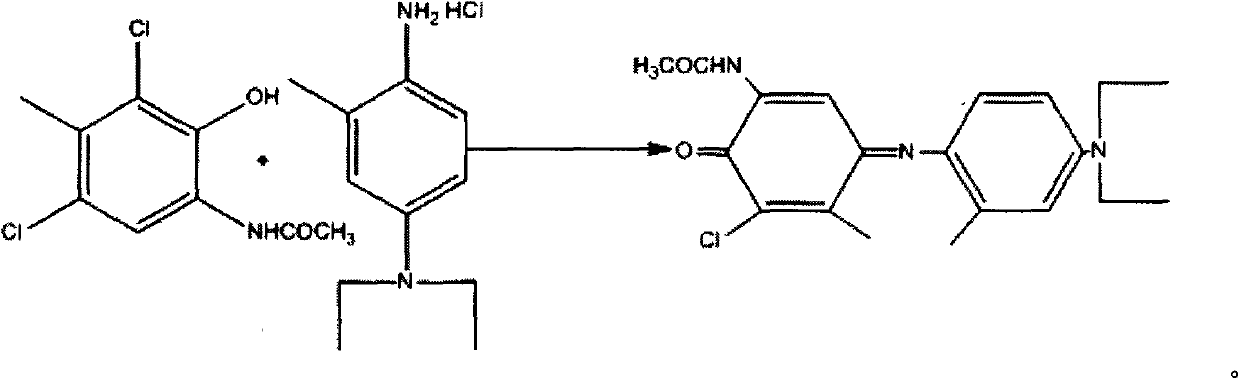
[0022] Then in a 2000ml three-necked flask, drop 2,4-dichloro-3-methyl 6-acetamidophenol 99.3g, compound 4 is 91.1g, (1.1eq.), methanol 1000ml, water 500ml, white suspension, Stir for 20min, the red color deepens, add 306.5g (6.8eq.) of sodium carbonate, stir for 20min, a gray clear solution, there is a gray clear solution at the bottom of the bottle, cool to the in...
Embodiment 2
[0024] Put 50g of 1, 400ml of acetonitrile, 21.5g (1.2eq.) of sodium acetate into a 500ml three-necked flask, add 25.7g (1.15eq.) of acetic anhydride dropwise at room temperature, filter, and wash with 80ml of water to obtain 49.4g of white solid compound 3. The yield was 96%. 50g of compound 3, 48.1g (1.05eq.) of compound 4, 500ml of methanol and water, white suspension, 113.2g (5eq.) of sodium carbonate added, deep red suspension, cold When the internal temperature was 0°C, 112g of sodium persulfate was added in batches, and the solution immediately turned dark red to blue. The addition was completed in 20 minutes, reacted for 3 hours, and filtered to obtain 68g of a red solid, which was recrystallized from ethyl acetate to obtain 48g of a bright red solid. The rate is 60%.
Embodiment 3
[0026] Put 27.2g of 1, 200ml of acetonitrile, 11.7g (1.1eq.) of sodium acetate into a 500ml three-necked flask, add acetic anhydride 13.4 (1.12eq.) dropwise at room temperature, filter with suction, and wash with 60ml of water to obtain 26g of compound 3 as a white solid. The yield was 93%. In a 500ml there-necked flask, 5g of compound 3, 4.7g (1.02eq.) of 4, 100ml of methanol and water, white suspension were added, 13.6g (6eq.) of sodium carbonate was added, and dark red suspension was cooled. When the internal temperature is 0°C, add 11.2 g of sodium persulfate in batches, and the solution turns dark red to blue immediately, and the addition is completed in 20 minutes. After reacting for 3 hours, filter to obtain 6.6 g of red solid, and recrystallize from ethyl acetate to obtain 6.2 g of bright red Solid, 80% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com