Preparation method and applications of olaquindox metabolite hapten
A technology for oquindox and metabolites, which is applied in the preparation methods of peptides, chemical instruments and methods, animal/human proteins, etc. The method is simple and feasible, the processing sample volume is large, and the cost is low.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, preparation and identification of olaquindox metabolite hapten
[0042] 1. Preparation of olaquindox metabolite hapten
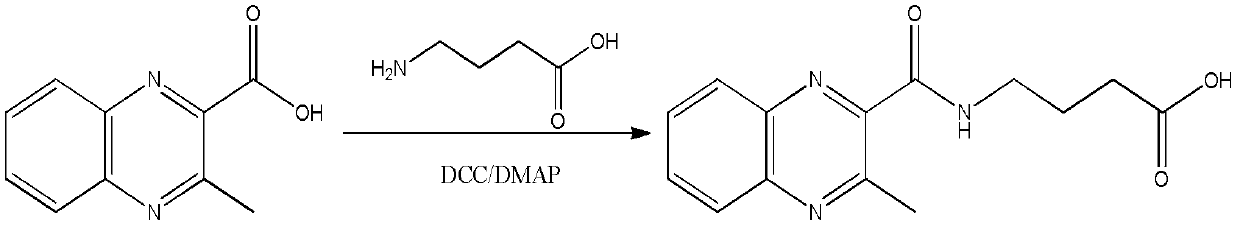
[0043] (1) Add 0.38g MQCA, 0.20g GABA and a small amount of DMAP to 20mL dry DMF;
[0044] (2) Slowly add 0.8 g of DCC in 5 mL of dry DMF mixture at 0°C. After the addition, the temperature is naturally raised to room temperature, and the reaction is continued for 40 h;
[0045] (3) The solvent was evaporated, and the condensation product of MQCA and aminobutyric acid was obtained after purification by column chromatography, which was the product shown in formula (1).
[0046] Second, the identification of olaquindox metabolite hapten
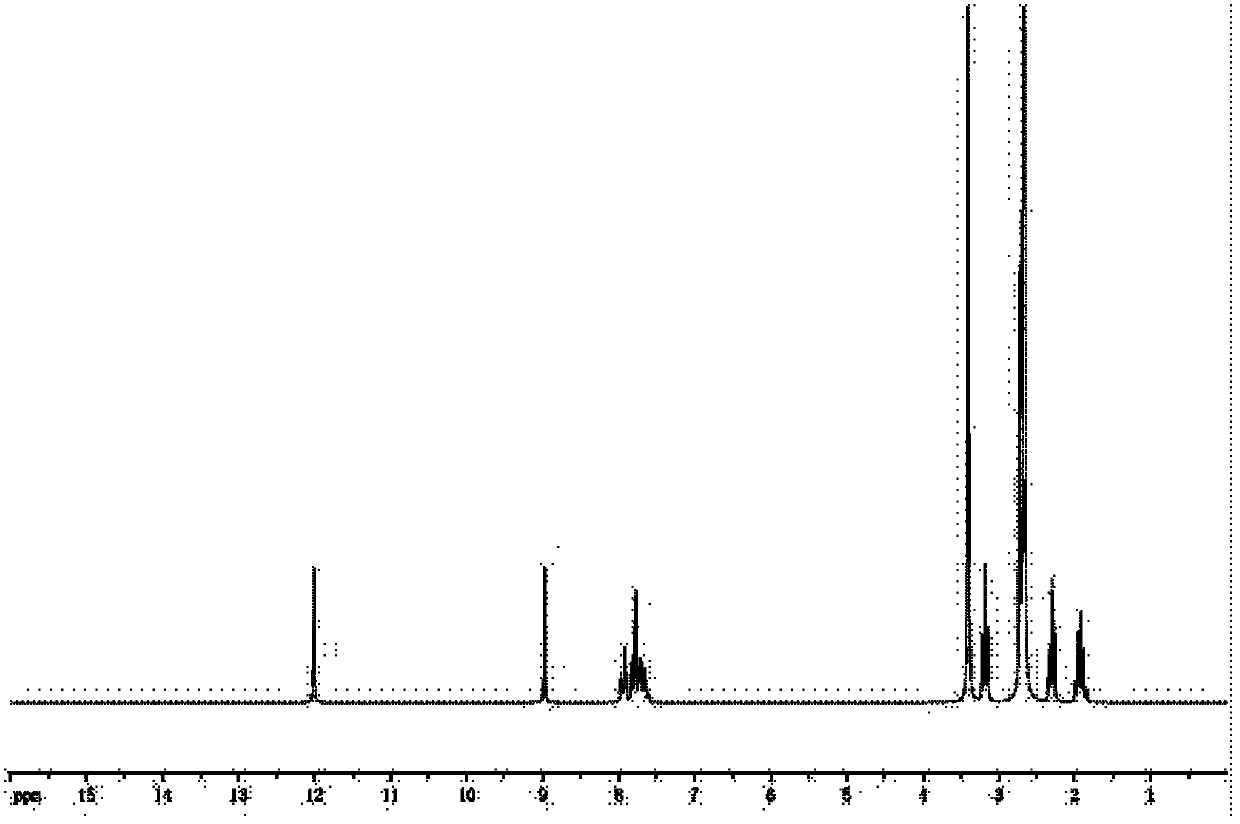
[0047] The NMR identification of the olaquindox metabolite hapten prepared in step one.
[0048] NMR see figure 2 . The spectrum shows a carboxyl signal peak around 12.0 and an increased methylene signal peak between 1.5 and 3.5, indicating that the hapten is successfully synthesized.
Embodiment 2
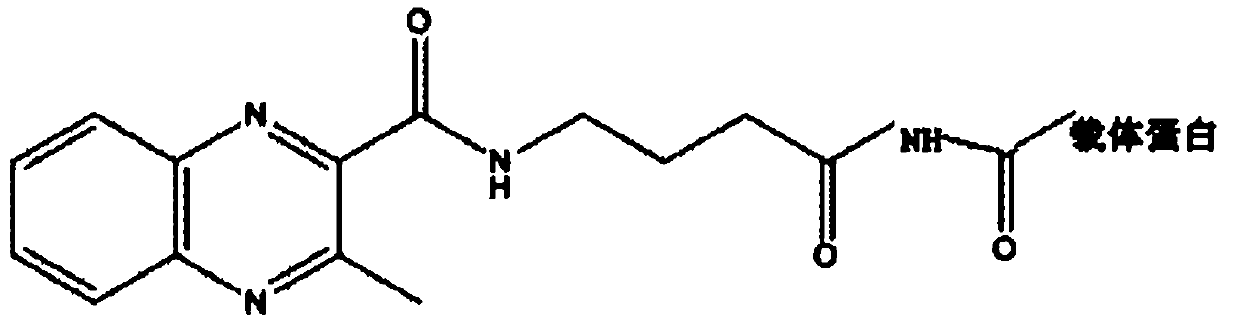
[0049] Embodiment 2, preparation and identification of olaquindox metabolite artificial antigen
[0050] 1. Synthesis of olaquindox metabolite immune antigen
[0051] (1) Fully dissolve 30 mg of the olaquindox hapten in 1 mL of DMF;
[0052] (2) Weigh 50 mg of BSA, make it fully dissolved in 3 mL of PBS (pH 7.2), slowly add the olaquindox hapten solution drop by drop to the BSA solution to obtain solution A;
[0053] (3) Weigh 12.5mg of EDC, dissolve it in 1mL of water, slowly add it to A at room temperature, and stir for 24h;
[0054] (4) Dialyze with 0.01mol / L PBS for 3 days, and change the dialysate twice a day to remove unreacted small molecular substances. Centrifuge at 12,000 rpm for 30 min, collect the supernatant, aliquot, and store at -20°C for later use.
[0055] 2. Synthesis of olaquindox metabolite-coated antigen
[0056] (1) Dissolve 20 mg of olaquindox hapten in 1.0 mL of DMF, cool to 10°C, add 15 μL of isobutyl chloroformate, and stir for 30 minutes at 10°C ...
Embodiment 3
[0062] Example 3, Preparation and Specific Identification of Monoclonal Antibody
[0063] 1. Preparation of olaquindox metabolite monoclonal antibody
[0064] 1. Use 100 μg of the immunogen (MQCA-BSA) prepared above, dissolve the immunogen in normal saline and mix it with Freund’s complete adjuvant in an equal volume, and inject subcutaneously on the back of the neck to immunize Balb / c females aged 6-8 weeks. On the 7th, 14th, and 28th day after the initial immunization, mix the immunogen and Freund's incomplete adjuvant in equal volumes, and each additionally immunize once, and 3 days before the fusion, 100 μg of the immune complex / rat, without Freund's adjuvant Add another immunization.
[0065] 2. Proceed according to the conventional method, take the splenocytes of the immunized mice and mix them with the mouse myeloma cells (SP2 / 0) in the logarithmic growth phase, and then slowly add the preheated fusion agent (PEG4000) within 45 seconds for fusion , suspend evenly with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com