Lyophilized vaccine for porcine rotavirus (PRV)
A porcine rotavirus and vaccine technology, which is applied in the directions of antiviral agents, viral antigen components, and medical preparations with non-active components, etc., to achieve the effects of low cost of raw materials, simple operation, and extended shelf life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
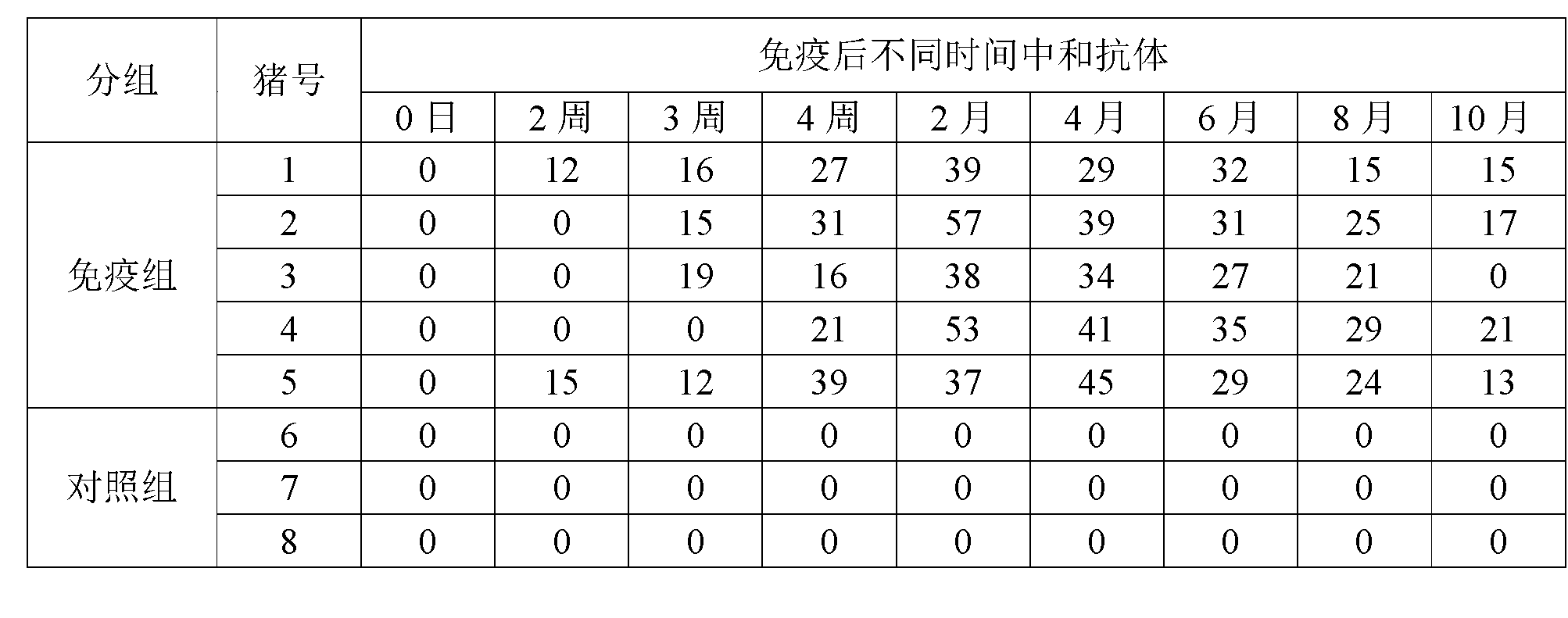
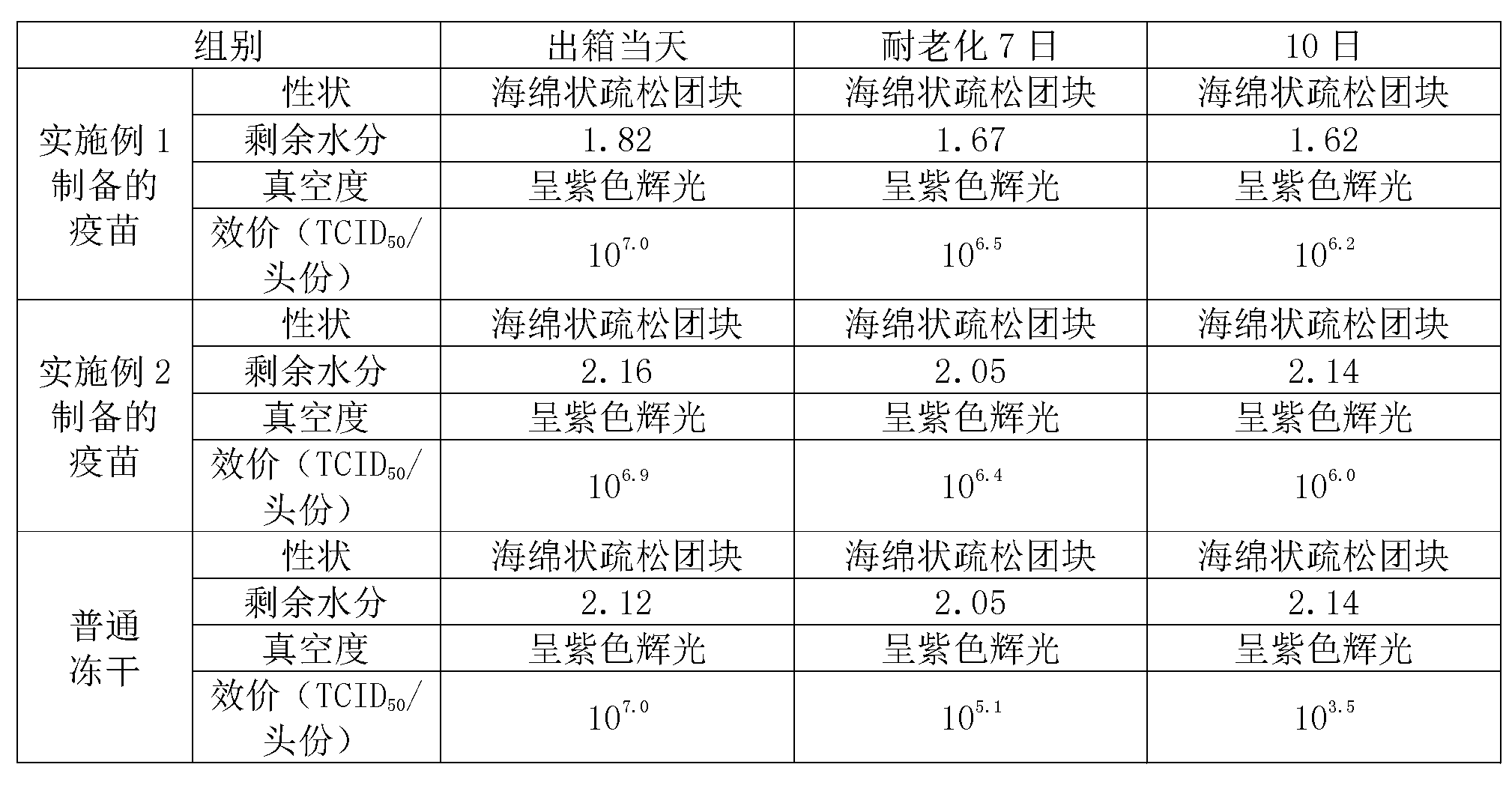
[0023] In the field of vaccine lyoprotectant preparation, the selection and ratio of the protective agent components have an important impact on the preservation effect of the vaccine. In order to enable the swine fever vaccine of the present invention to be preserved for a long time, the applicant has carried out long-term research on the components of the lyoprotectant of the present invention, and optimized the proportioning concentrations of each other, so that each component has the greatest complex matching effect.
[0024] 1. Preparation of lyoprotectant: divided into two parts, liquid A and liquid B.
[0025] 1) Preparation of liquid A: Weigh 48g of NZ-amine, 6g of monopotassium glutamic acid, 400g of sucrose, and 30g of hydrolyzed milk protein; dissolve the above ingredients in 800ml of water for injection in order, shake well after fully dissolving, and add water for injection After reaching 1000ml, shake well, filter and sterilize with a 0.22μm filter membrane, and...
Embodiment 2
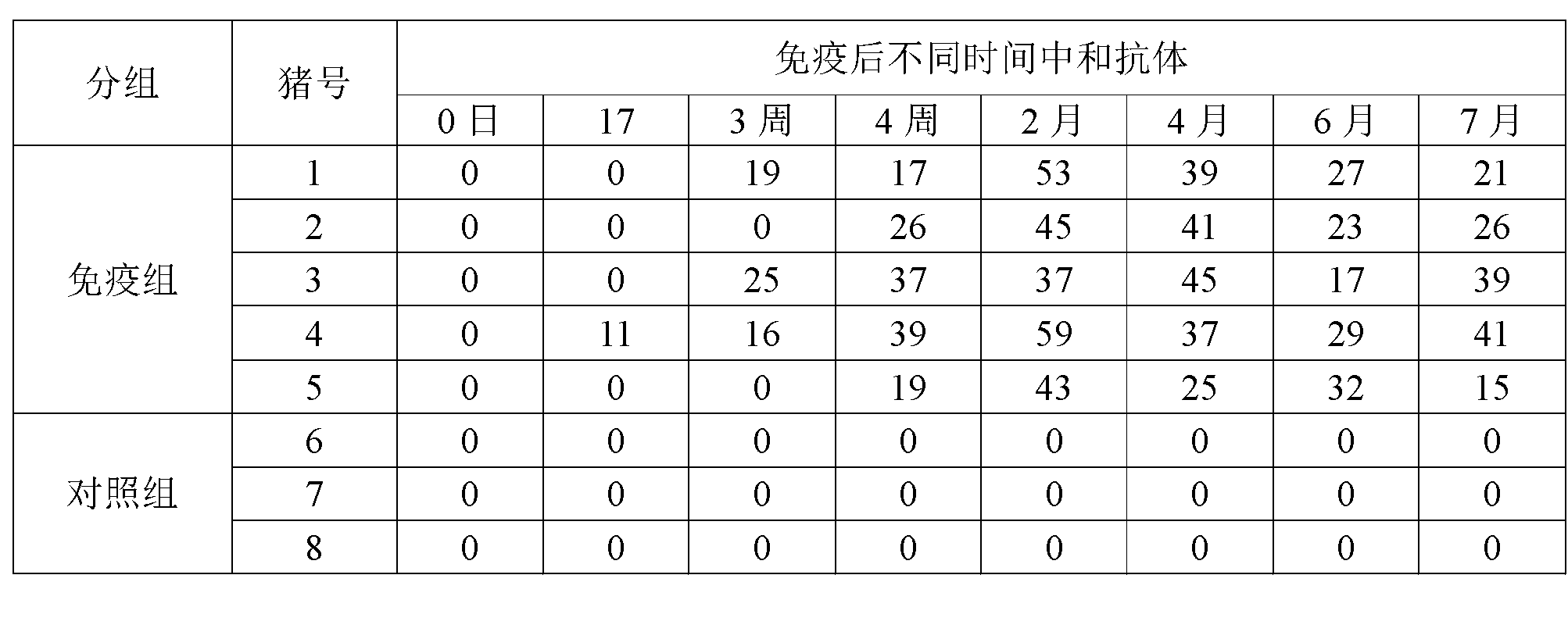
[0035] 1. Preparation of lyoprotectant:
[0036] Preparation of lyoprotectant: divided into two parts, liquid A and liquid B.
[0037] 1) Preparation of liquid A: Weigh 80g of NZ-amine, 10g of monopotassium glutamic acid, 500g of sucrose, and 50g of hydrolyzed milk protein; dissolve the above ingredients in 800ml of water for injection in order, shake well after fully dissolving, and add water for injection to 1000ml, sterilized by filtration with a 0.22μm filter membrane, and stored in a 37°C greenhouse for future use.
[0038] 2) Preparation of liquid B: Take 120g of gelatin, fully dissolve it with 800ml of water for injection at 70-80°C, add water for injection to 1000ml, autoclave at 121°C and 15 pounds for 30 minutes, and store at 37°C after sterilization.
[0039] 3) Proportion according to the volume ratio of liquid A: liquid B = 1:1, and mix well to get the heat-resistant freeze-drying protective agent.
[0040] 2. Vaccine preparation
[0041] 1. After porcine rotav...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com