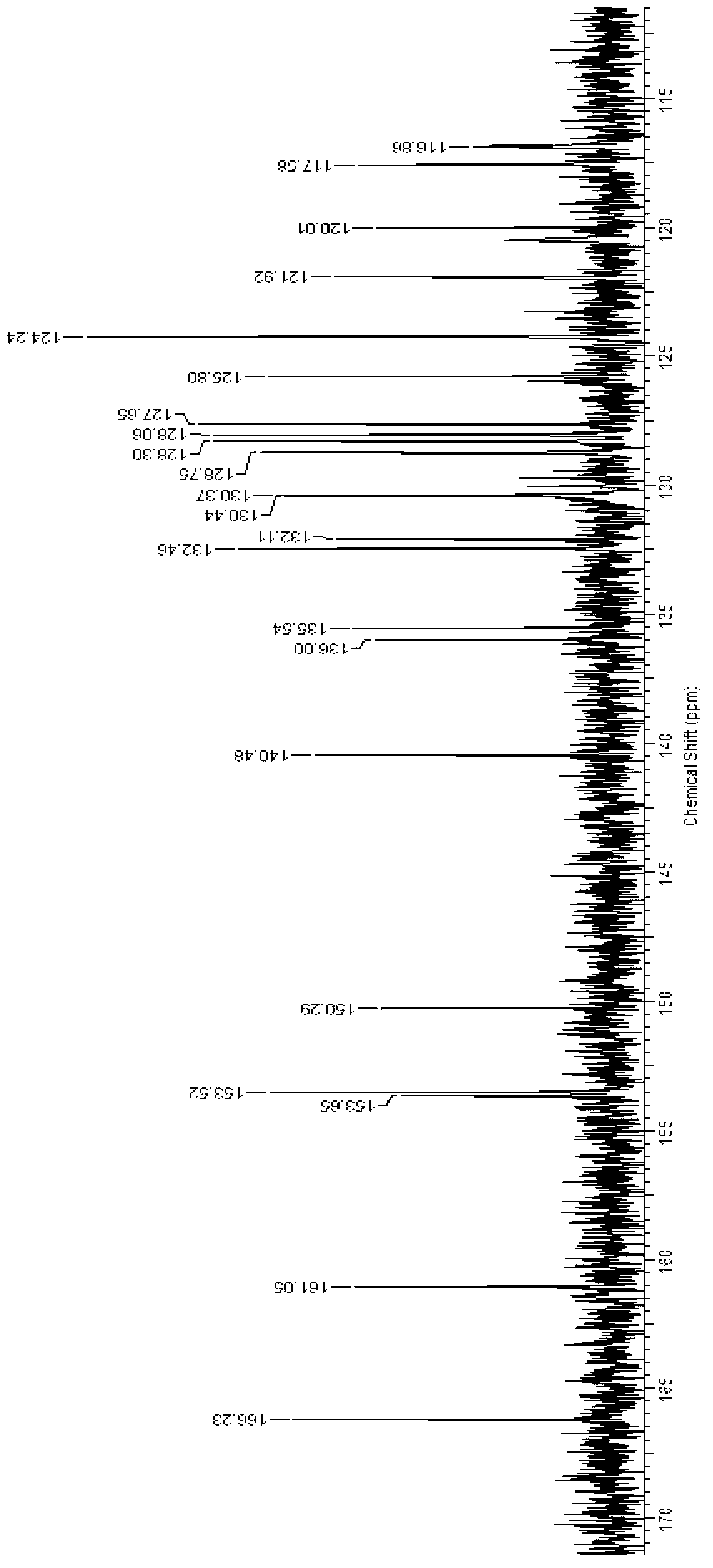
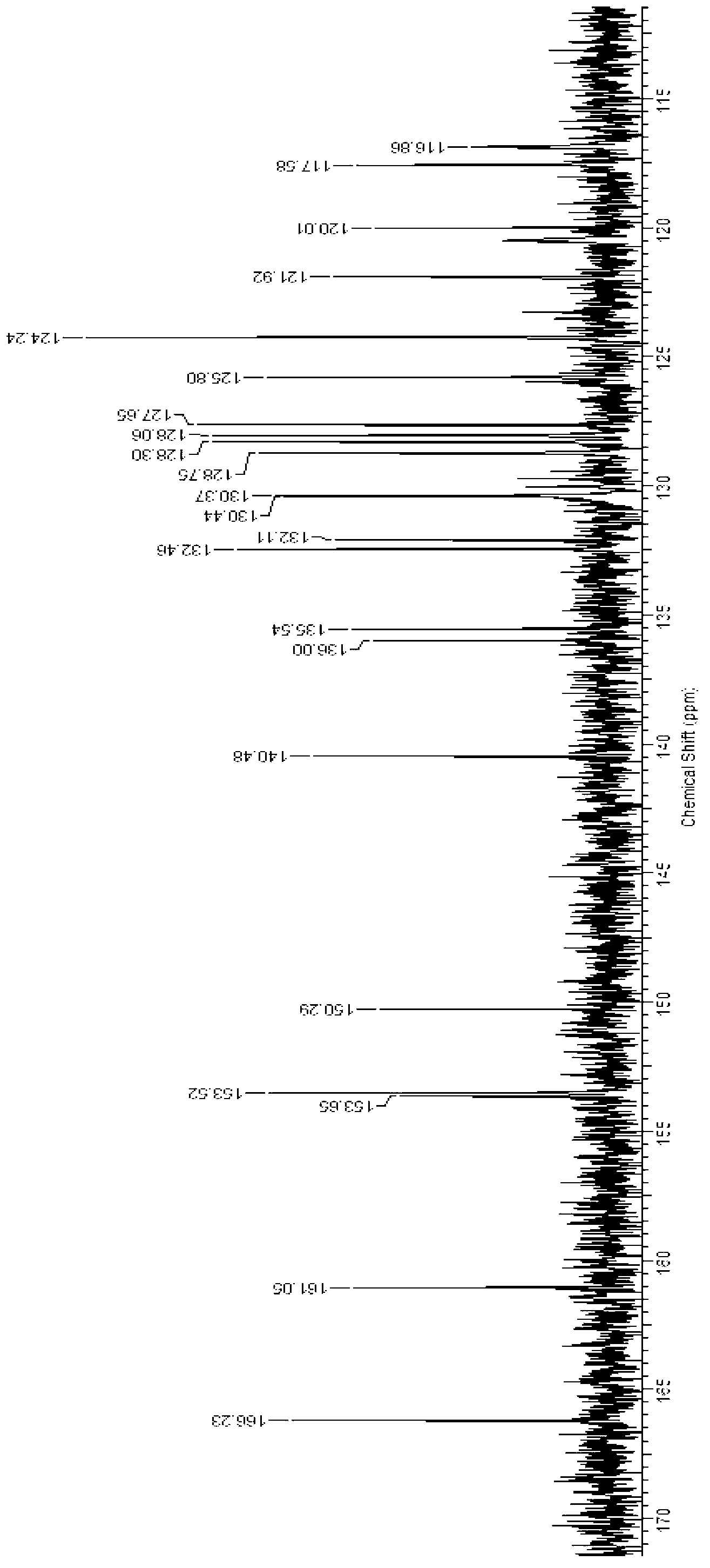
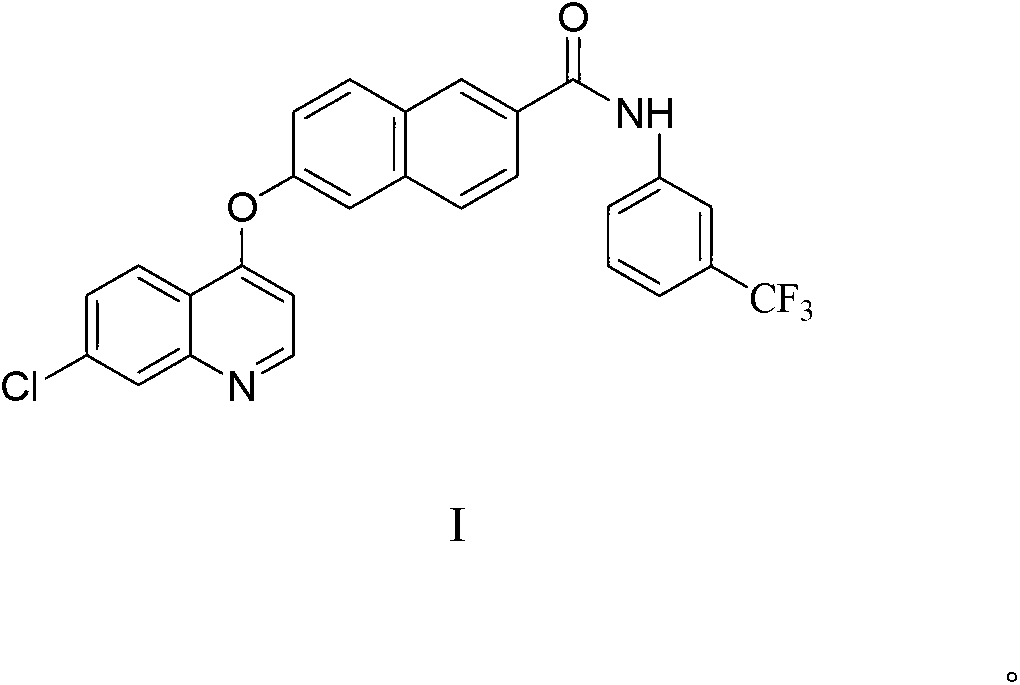
Naphthoyl amine derivative, and preparation method and application thereof
A technology for naphthylcarboxamide and derivatives, applied in the field of naphthylcarboxamide derivatives, can solve the problems of low yield, unsuitable industrial production, high price, etc., achieve high yield, simple and easy control of the reaction process, and reduce reaction cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present invention also relates to a kind of preparation method of naphthylcarboxamide derivative, described preparation method is as follows:
[0044] Step 10. Dissolve compound II in ethanol, then add concentrated sulfuric acid dropwise, heat and reflux at 78-85°C for 4-5 hours, distill off the solvent under reduced pressure, add ethyl acetate, then use saturated sodium bicarbonate and saturated chlorine NaCl was washed 2-3 times respectively, the organic phases were combined, and the solvent was distilled off under reduced pressure to obtain compound III. The reaction equation is as follows:
[0045]
[0046] Wherein, compound II is 6-hydroxyl-2-naphthoic acid, and compound III is 6-hydroxyl-2-naphthoic acid;
[0047] Step 20. Dissolve the obtained compound III in DMF, stir in the ice bath, slowly add the DMF solution containing NaH dropwise, stir in the ice bath for half an hour, remove the ice bath, wait until the temperature returns to room temperature, slowly...
Embodiment 1
[0056] Compound 6-hydroxyl-2-naphthoic acid 1.88g (10mmol) was dissolved in 40 milliliters of ethanol, added 4 drops of concentrated sulfuric acid, heated to reflux at 80°C for 5 hours, and then the solvent was removed under reduced pressure to obtain compound 6-hydroxyl-2- Ethyl naphthoate 1.98g (9.2mmol), yield 91.7%;
[0057] The obtained compound 6-hydroxyl-2-naphthoic acid ethyl ester 1.98g (9.2mmol) was dissolved in 5 milliliters of DMF, stirred under ice bath, slowly added dropwise the DMF solution 10 milliliters containing 0.52g (21.7mmol) of NaH, ice The bath was stirred for half an hour, the ice bath was removed, and when the temperature returned to room temperature, 10 ml of DMF solution containing 2.19g (11.1mmol) 4,7-dichloroquinoline and 0.38g (1.8mmol) KI was slowly added dropwise, and stirred for half an hour , reacted at 110° C. for 8-10 hours, then removed the solvent under reduced pressure to obtain the reaction mixture, separated by silica gel column chroma...
Embodiment 2
[0064] Compound 6-hydroxyl-2-naphthoic acid 3.53g (20mmol) was dissolved in 40 milliliters of ethanol, added 8 drops of concentrated sulfuric acid, heated to reflux at 80°C for 5 hours, and then the solvent was removed under reduced pressure to obtain compound 6-hydroxyl-2- Ethyl naphthoate 4.01g (18.5mmol), yield 92.5%;
[0065] Dissolve 2.01 g (18.5 mmol) of the compound 6-hydroxyl-2-ethyl naphthoate in 5 mL of DMF, stir in an ice bath, slowly add 15 mL of a DMF solution containing 1.02 g (41.7 mmol) of NaH, and place in an ice bath Stir for half an hour, remove the ice bath, wait for the temperature to return to room temperature, slowly drop into 15 ml of DMF solution containing 3.68g (18.6mmol) 4,7-dichloroquinoline, and 0.69g (4.16mmol) KI, stir for half an hour, React at 110°C for 8-10 hours, then remove the solvent under reduced pressure to obtain the reaction mixture, which is separated by silica gel column chromatography, the gradient of the mobile phase is: V (n-hexa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com