Method for co-producing adipic acid and nitrocyclohexane
A technology of nitrocyclohexane and cyclohexane, which is applied in the direction of chemical instruments and methods, preparation of nitro compounds, carboxylate preparation, etc., can solve the problems of high cost, difficult separation of soluble catalysts, low selectivity of adipic acid, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
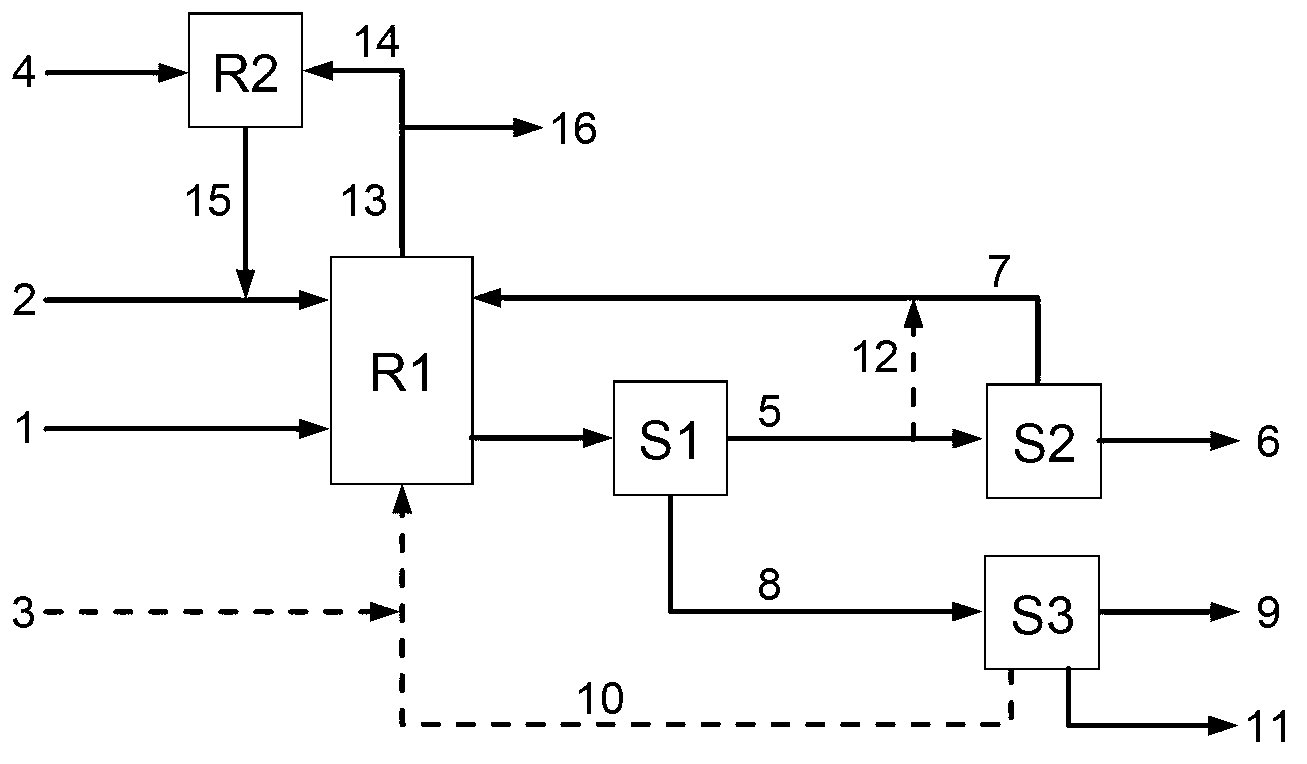
[0078] Embodiment 1: press cyclohexane and NO 2 The molar ratio of cyclohexane to catalyst is 0.2:1, and the mass ratio of cyclohexane to catalyst is 1:0.1. Add 5.04g cyclohexane and 13.80g liquid NO to a 0.2L autoclave 2 And 0.54g catalyst Cr-AlVPO; react at 80°C and 2.0MPa for 24h under stirring conditions. Stratification after standing and cooling, the upper layer is an oil phase, mainly containing unreacted cyclohexane and product nitrocyclohexane, the lower layer is mainly a mixture of adipic acid crystals, catalyst solids, and reaction water; filter all materials And wash the filter cake with quantitative cyclohexane and distilled water respectively; The collected filtrate and washing liquid are separated to obtain the oil phase and the water phase and are measured respectively. The oil phase is quantitatively determined by gas chromatography internal standard method. The composition was determined by liquid chromatography external standard method; the filter cake was d...
Embodiment 2
[0079] Embodiment 2: As in Embodiment 1, the difference lies in that the reaction temperature is 100° C., and the reaction pressure is 2.9 MPa. The conversion rate of cyclohexane was 75.4%, the selectivity of adipic acid was 81.7%, the selectivity of nitrocyclohexane was 11.5%, and the sum of the two selectivities was 93.2%.
Embodiment 3
[0080] Embodiment 3: as embodiment 1, by cyclohexane and NO 2 Add 8.40g cyclohexane and 4.60g liquid NO at a molar ratio of 1:1.0 2 , the catalyst is VPO; the reaction temperature is 60°C, and the reaction pressure is 0.6MPa. The conversion rate of cyclohexane was 11.1%, the selectivity of adipic acid was 80.6%, the selectivity of nitrocyclohexane was 11.9%, and the sum of the two selectivities was 92.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com