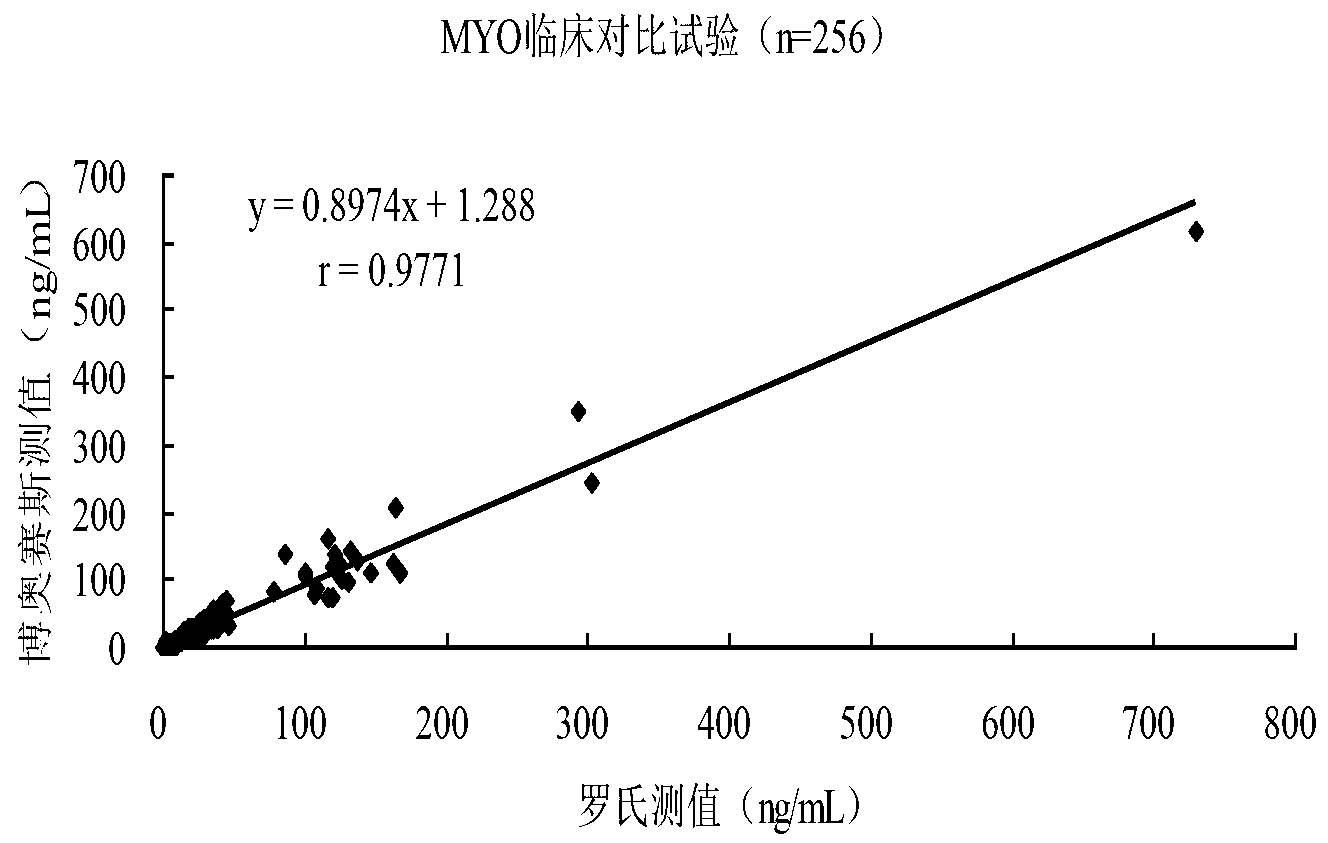
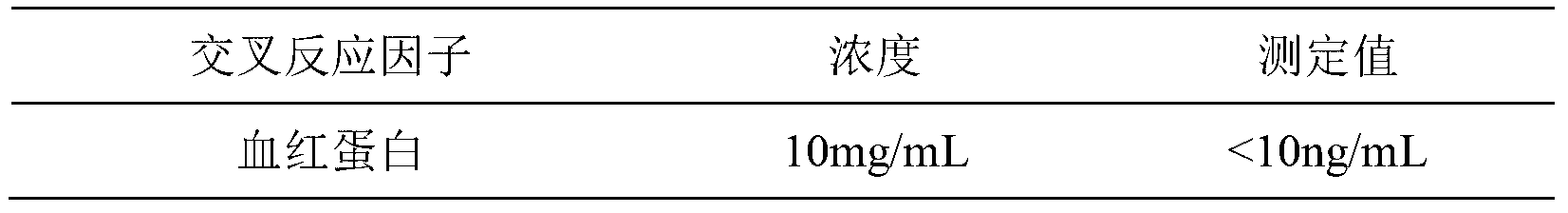
Kit for chemiluminescence immunity quantitative detection of MYO (myohaemoglobinnano) nano magnetic particle and preparation method of kit
A technology of chemiluminescent immunity and nano-magnetic particles, which is applied in the field of immunoanalysis medicine, can solve the problems of narrow detection range, inaccurate quantification of colloidal gold test strips, and poor sensitivity of enzyme-linked immunoassay, achieving good stability and low cost , good specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Preparation of Myoglobin Nano Magnetic Particle Chemiluminescent Immunoquantitative Assay Kit
[0059] (1) Preparation of myoglobin calibrator:
[0060] The myoglobin antigen (purchased from Fitzgerald Company) was prepared with goat serum (Zhengzhou Yikang Bioengineering Co., Ltd.) to make a concentrated stock solution of calibrator, and the enterprise standard was used for calibration, and the concentrated stock solution was diluted to the working concentration with goat serum , respectively 0, 5, 25, 100, 250, 1000ng / mL;
[0061] (2) Preparation of myoglobin control product:
[0062] Dilute the above concentrated stock solution to 10ng / mL and 500ng / mL with goat serum, use 10ng / mL as a low-value quality control, and 500ng / mL as a high-value quality control;
[0063] (3) Preparation of magnetic nanoparticle-streptavidin suspension:
[0064] A. Preparation of ferroferric oxide nano-magnetic particles
[0065] Precipitation method was used to prepare ferro...
Embodiment 2
[0081] Embodiment 2: the inspection of kit of the present invention
[0082] (1) Physical inspection: liquid components should be clear without sediment or floc; other components should have no package damage.
[0083] (2) Accuracy: The kit calibrator and the national standard series are analyzed and measured at the same time, and the double-logarithmic mathematical model is used to fit the two dose-response curves. It is required that the two dose-response curves do not deviate significantly from parallel (t test, |t|<2.447) ; Take the myoglobin enterprise standard as the reference substance, and use the double-logarithmic mathematical model to fit, and the average value of the ratio between the measured value and the labeled value of the kit calibrator should be within the range of 0.90 to 1.10.
[0084] (3) Linearity of the dose-response curve: Fitting with a double-logarithmic mathematical model, the absolute value of the correlation coefficient r of the dose-response curv...
Embodiment 3
[0091] Embodiment 3: the using method of kit of the present invention
[0092] (1) Equilibrate the test kit at room temperature (18-25°C) for 30 minutes.
[0093] (2) Preparation of lotion: dilute the concentrated lotion with distilled water at 1:20 (1mL of lotion plus 19mL of distilled water). If the concentrated lotion has crystals, the concentrated lotion can be placed at room temperature or 37°C, and then diluted after the crystals dissolve.
[0094] (3) Preparation of luminous liquid: Take an appropriate amount of luminous liquid A and luminous liquid B and mix them in equal volumes 5 minutes before use.
[0095] (4) Number the reaction tubes, add 25uL calibrator or serum sample, 50uL magnetic particle-streptavidin suspension, 50uL biotin-myoglobin antibody conjugate, 100uL myoglobin antibody enzyme conjugate to the test tube Shake and react at 37°C for 30 minutes, place the test tube rack on a magnetic separator for separation for 5 minutes, then pour out the supernata...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com