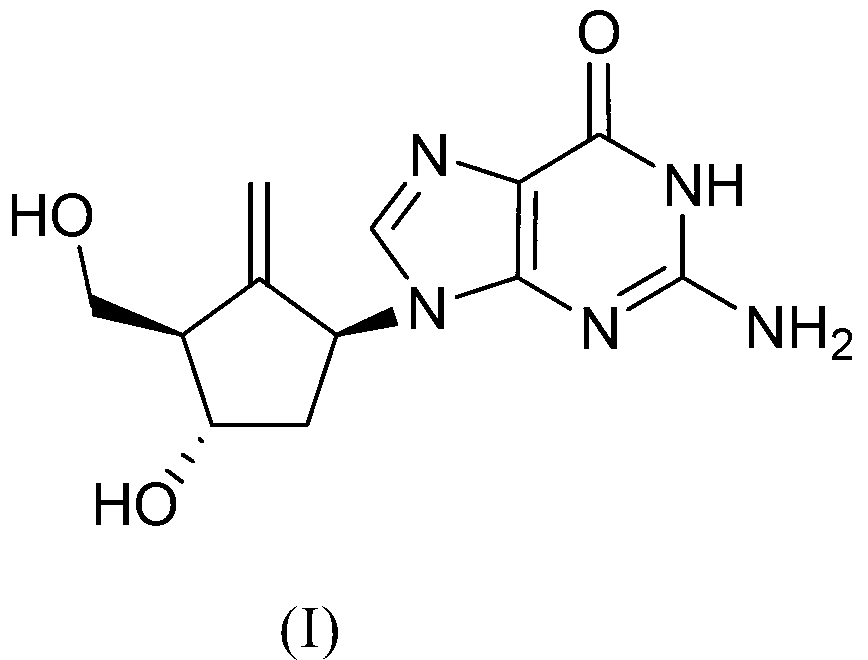
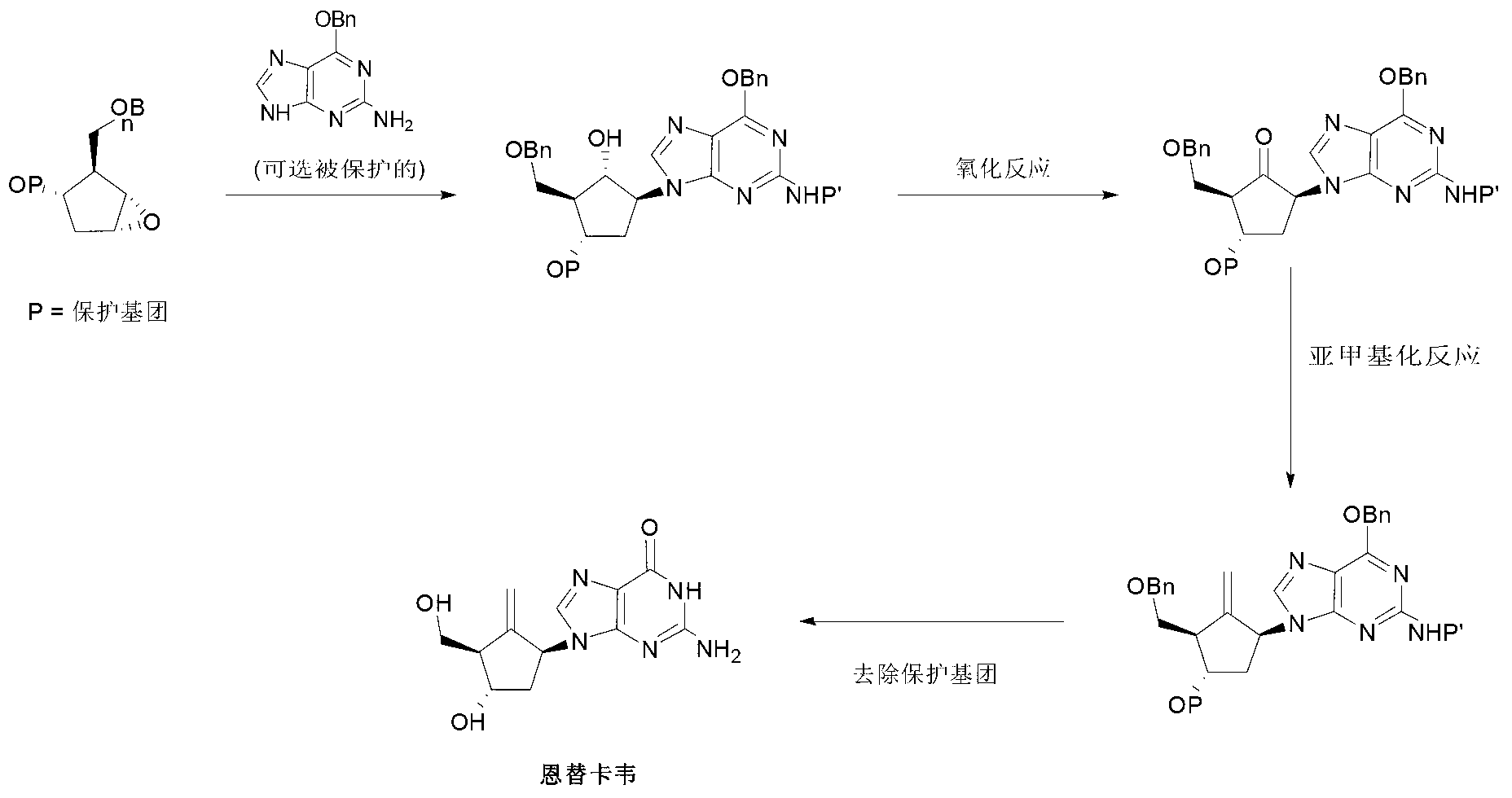
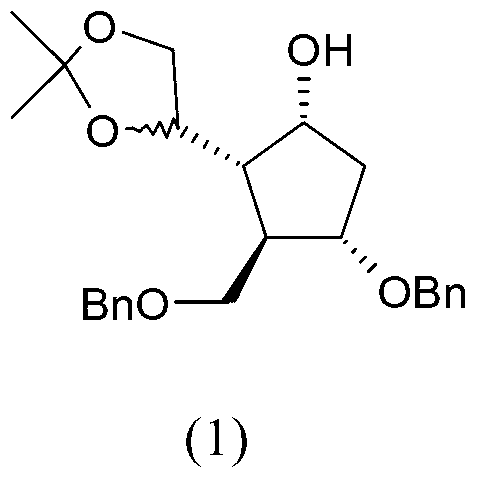
Preparation process of an antiviral drug (entecavir) and intermediates thereof
A technology for entecavir and medicine, which is applied in the preparation of antiviral medicine (entecavir) and the field of intermediates thereof, and can solve the problems such as difficulty in utilizing known methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1: (3S,5R)-7-(Trimethylsilyl)hept-1-en-6-yne-3,5-diol (XI) preparation of
[0123] at 0°C, N 2 To a solution of (+)-DIPCl (90-105%) (25 g, 77.94 mmol) in anhydrous tetrahydrofuran (THF) (40 mL) was added triethylamine (NEt 3 ) (85.02 mmol, 11.6 mL). Then, 98% 4-trimethylsilyl-3-butyn-2-one (70.85 mmol, 9.78 g) was added dropwise, and the reaction mixture was stirred at -5°C-0°C for 2 h. The solution was then cooled to –78°C. A solution of acrolein (90% purity) (102.6 mmol, 7.62 mL) in anhydrous THF (20 mL) was added slowly, and the reaction mixture was stirred at -78 °C for 1 h. Slowly add lithium borohydride (LiBH 4 ) 2M solution in hexane (106.28 mmol, 53 mL). The reaction mixture was stirred for an additional 1 h at −78 °C, then carefully washed with NH 4 A saturated solution of Cl (40 mL) was quenched for 0.5 h. (temperature increased from –78°C to room temperature). H 2 O (40 mL) and tert-butyl methyl ether (TBME) (90 mL) were partitioned. The...
Embodiment 2
[0126] Example 2: (3S,5R)-5-(tert-butyldimethylsilyloxy)-7-(trimethylsilyl) Preparation of Hept-1-en-6-yn-3-ol (X)
[0127] at 0°C, N 2 To a solution of diol (XI) (5.0 g, 25.2 mmol) and imidazole (2.1 g, 30.3 mmol) in anhydrous THF (60 mL) was added dropwise anhydrous THF (20 mL) with TBSCl (4.23 g, 27.7 mmol) under atmosphere. ) solution, and the reaction mixture was warmed to room temperature. The reaction mixture was stirred for 5h. Then, slowly add 22% NH 4 Cl solution (25 mL), and the reaction mixture was stirred for 10 min. The mixture was partitioned and the organic phase was dried (MgSO 4 ), and the volatiles were removed in vacuo. The resulting oily material was then purified by silica gel flash chromatography (hexane-ethyl acetate 90:10 to 80:20) to yield 4.84 g (61%) of the title compound. Pale yellow oil. Rf (Hexane:AcOEt 80:20)=0.55. [α] D =+39.9(c1.0, CHCl 3 ). IR (film) (cm -1 ): 3424, 3081, 2958, 2172.
Embodiment 3
[0128] Example 3: (3S,5R)-5-(tert-butyldimethylsilyloxy)hept-1-en-6-yn-3-ol Preparation of (IXa)
[0129] at room temperature, N 2 K 2 CO 3 (0.101 g, 0.73 mmol) was added in one portion to a stirred solution of (X) (0.455 g, 1.46 mmol) in dry methanol (MeOH) (4.5 mL). The reaction mixture was stirred for 1 h. After the reaction was complete, the volatiles were removed, and CH was added to the residue 2 Cl 2 (10 mL). The mixture was filtered, dried (MgSO 4 ), and removal of volatiles yielded 0.366 g (yield: 100%) of the title compound. Pale yellow oil. Rf (Hexane:AcOEt 80:20)=0.43. [α] D =+32.7(c1.0, CHCl 3 ). IR (film) (cm -1 ): 3417, 3079, 2956, 2109.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com