Novel <18>F marked 4-aminoquinazoline derivatives, and preparation methods and tumor PET development application thereof
A technology of aminoquinazolines and quinazolines, which is applied in the field of new 18F-labeled 4-aminoquinazoline derivatives and its preparation and tumor PET imaging applications, which can solve the problem of poor stability in vivo and low tumor uptake , Poor water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
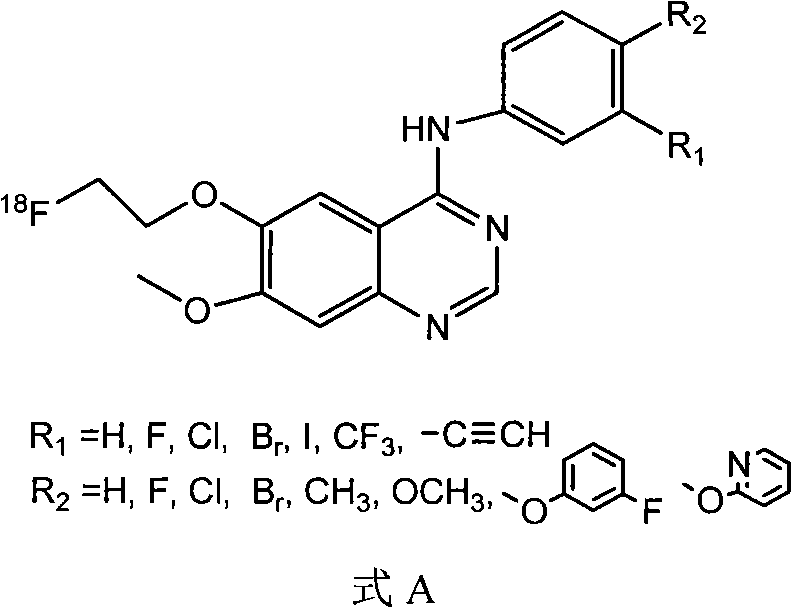
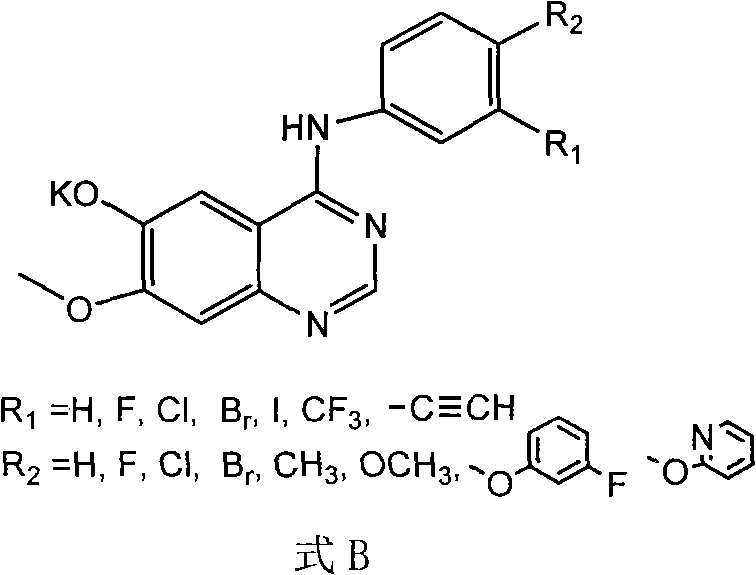
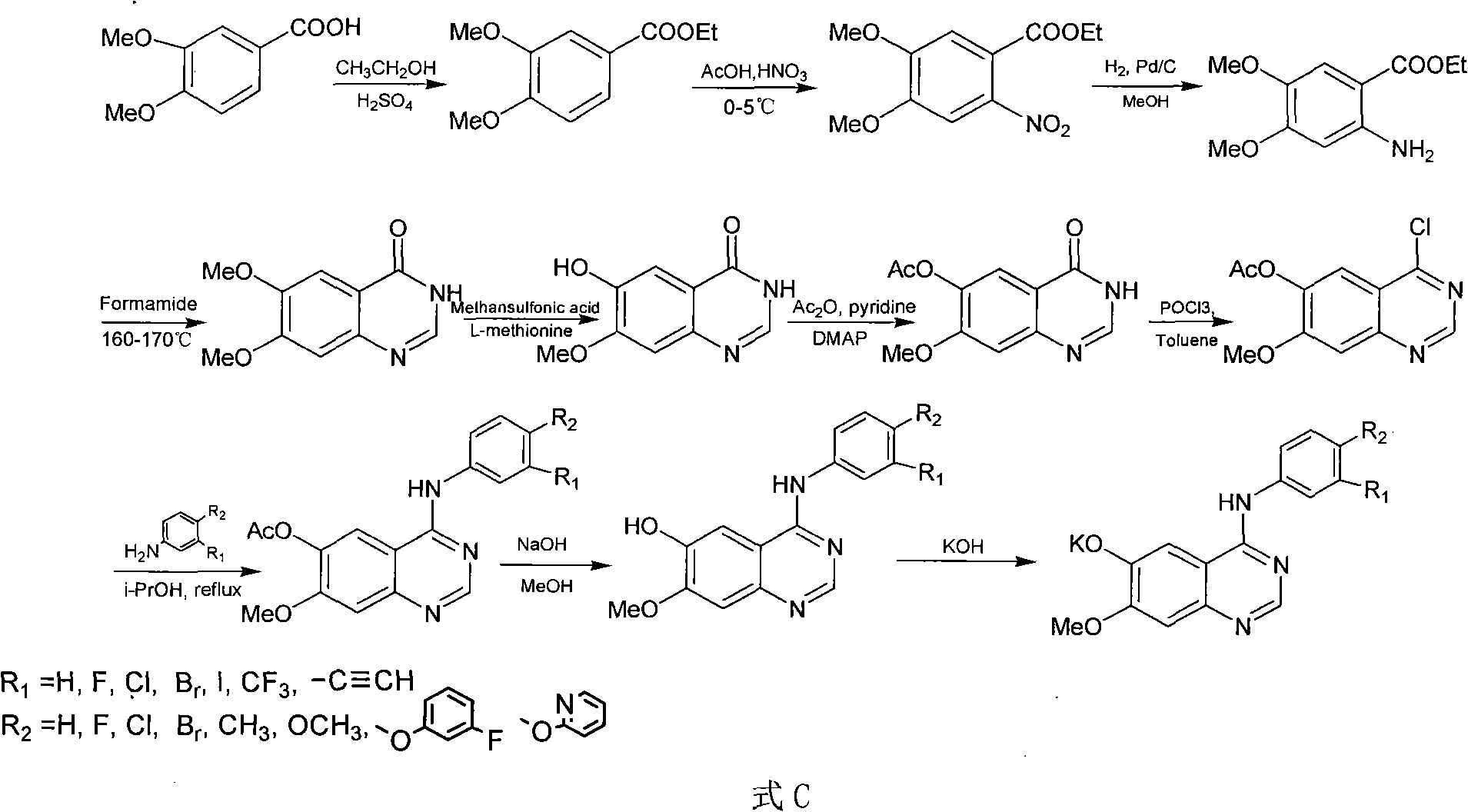
[0081] Prepared according to the following steps is R in formula A 1 =Br,R 2 =H compounds, including labeling precursors (R in formula B 1 =Br,R 1 =H compounds) organic synthesis and radiochemical synthesis of two parts.
[0082] 1) labeled precursor (R in formula B 1 =Br, R 2 = Compound of H)
[0083] 1.1 Synthesis of ethyl 3,4-dimethoxybenzoate
[0084] Add 3,4-dimethoxybenzoic acid (10g, 0.055mol) and ethanol (100mL) into a 250mL reaction flask, and slowly add concentrated sulfuric acid (8mL) dropwise under ice bath, heat up, and heat to reflux for 8-10h, TLC tracking monitoring, the reaction is complete, decompression spin off ethanol, add 100mL distilled water, use K 2 CO 3 The pH was adjusted to neutral, extracted with ethyl acetate, the organic layer was collected, and the solvent was spin-dried to obtain a pale yellow oil with a yield of 92%. 1 H-NMR (CDCl 3, 500MHz) δ (ppm): 7.65-7.67 (dd, 1H, Ar-H), 7.58-7.59 (d, 1H, Ar-H), 6.09-6.91 (d, 1H, Ar-H), 4.32-4.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com