Application of mitiglinide in preparation of medicine for treating cerebral arterial thrombosis
A technology for ischemic stroke and ischemic stroke, applied in the field of application of mitiglinide in the preparation of drugs for the treatment of ischemic stroke, achieving huge market value and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Neuroprotective effect of mitiglinide on transient middle cerebral artery embolism model (tMCAo) mice
[0024] 1.1 Experimental materials
[0025] 1.1.12-3 months old, male C57BL / 6J mice, 24-28g. Purchased from the Experimental Animal Center of Nanjing Medical University, animal production license number: SCXK (Su) 2008-0004. Feeding conditions include standard feed, tap water, room temperature maintained at (24±2)°C, humidity 50-60%, and daily light and dark times of 12 hours each. Before the experiment, the animals were placed in the experimental environment for 3 days to adapt.
[0026] 1.1.2 Miglinide calcium (Lianyungang Runzhong Pharmaceutical Co., Ltd., batch number 1612001), prepared with normal saline, the concentration of the mother solution is 1.2mg / ml, and stored at -20°C. 2,3,5-triphenyltetrazolium chloride (2,3,5-triphenyltetrazoliumchloride, TTC, purchased from sigma company), and others are commercially available.
[0027] 1.2 Method
[00...
Embodiment 2
[0037] Example 2 Study on the neuroprotective effect of mitiglinide on rats with permanent middle cerebral artery embolism (pMCAo)
[0038] 2.1 Experimental materials
[0039] 2.1.1 Animals: 2-3 months old, male SD rats, 230-270g. Purchased from the Experimental Animal Center of Nanjing Medical University, animal production license number: SCXK (Su) 2008-0004. Feeding conditions include standard feed, tap water, room temperature maintained at (24±2)°C, humidity 50-60%, and daily light and dark times of 12 hours each. Before the experiment, the animals were placed in the experimental environment for 3 days to adapt.
[0040] 2.1.2 Same as 1.1.2.
[0041] 2.2 Method
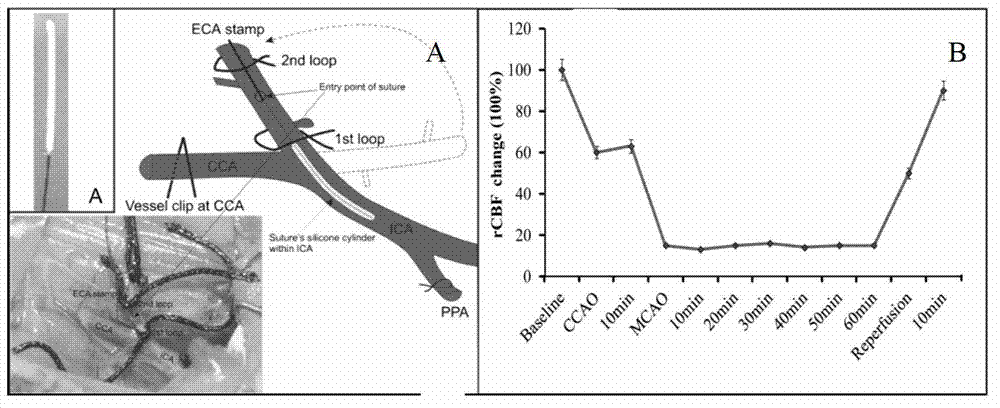
[0042] pMCAo rat model: Rats were fasted for 12 hours before operation, and the rats were anesthetized by intraperitoneal injection of chloral hydrate (350mg / kg), so that the rats stopped their voluntary activities and their muscles relaxed. Referring to the tMCAo mouse model, the right middle cerebral artery ...
Embodiment 3
[0047] Implementation Example 3 The protective effect of mitiglinide on oxygen-glucose deprivation SH-SY5Y cell injury
[0048] 3.1 Experimental materials
[0049] SH-SY5Y cell line: neuron cell line, purchased from Union Cell Institute, Chinese Academy of Medical Sciences. Methazolium Blue (MTT) was purchased from Guangzhou Chemical Reagent Factory; microplate reader was purchased from Thermo Company.
[0050] LDH assay kit: product of Nanjing Jiancheng Institute of Bioengineering.
[0051] 3.2 Method
[0052] 3.2.1 SH-SY5Y cell oxygen-glucose deprivation model: SH-SY5Y cell line was cultured in DMEM medium containing 10% FBS (37°C, 5% CO 2 ), inoculated on a 96-well plate and cultured for 24 hours before the experiment. On the experimental day, pre-administration was incubated for 30 minutes, and then the drug-containing sugar-free DMEM medium was changed to a three-gas incubator (37°C, 1% O 2 , 94%N 2 , 5%CO 2 ) Oxygen-glucose-deprived culture for 60 minutes, return ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com