Cancer cell-targeting structural molecule and use thereof
A targeting, cancer cell technology, applied in the field of biomedicine, can solve problems such as poor targeting and difficult preparation, and achieve the effect of increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] 1. Synthesis of RGD-PEG-MPA-siRNA VEGFR2 molecule.
[0065] Synthetic route such as figure 1 As shown, the specific steps are as follows:
[0066] 1.1. RGD-PEG-MPA (Tumor Targeting Structural Molecule)
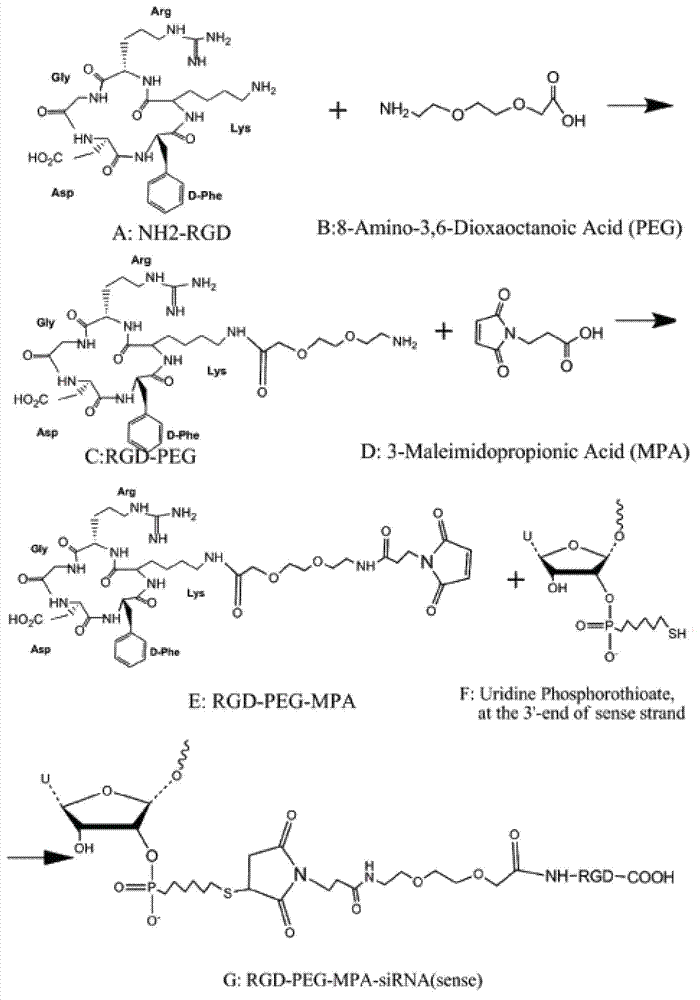
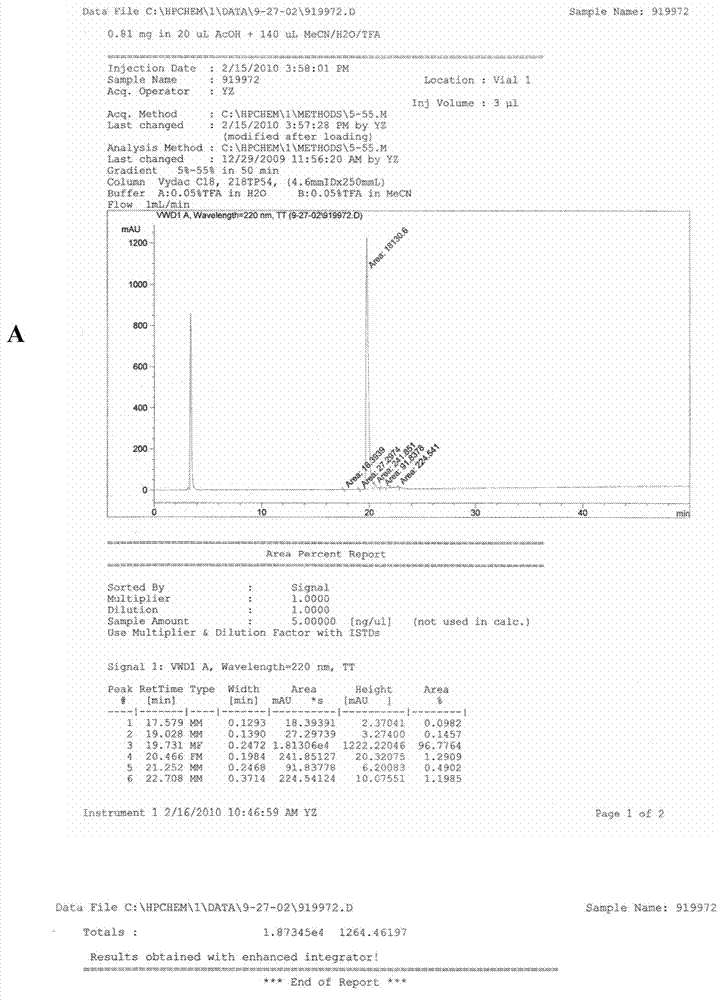
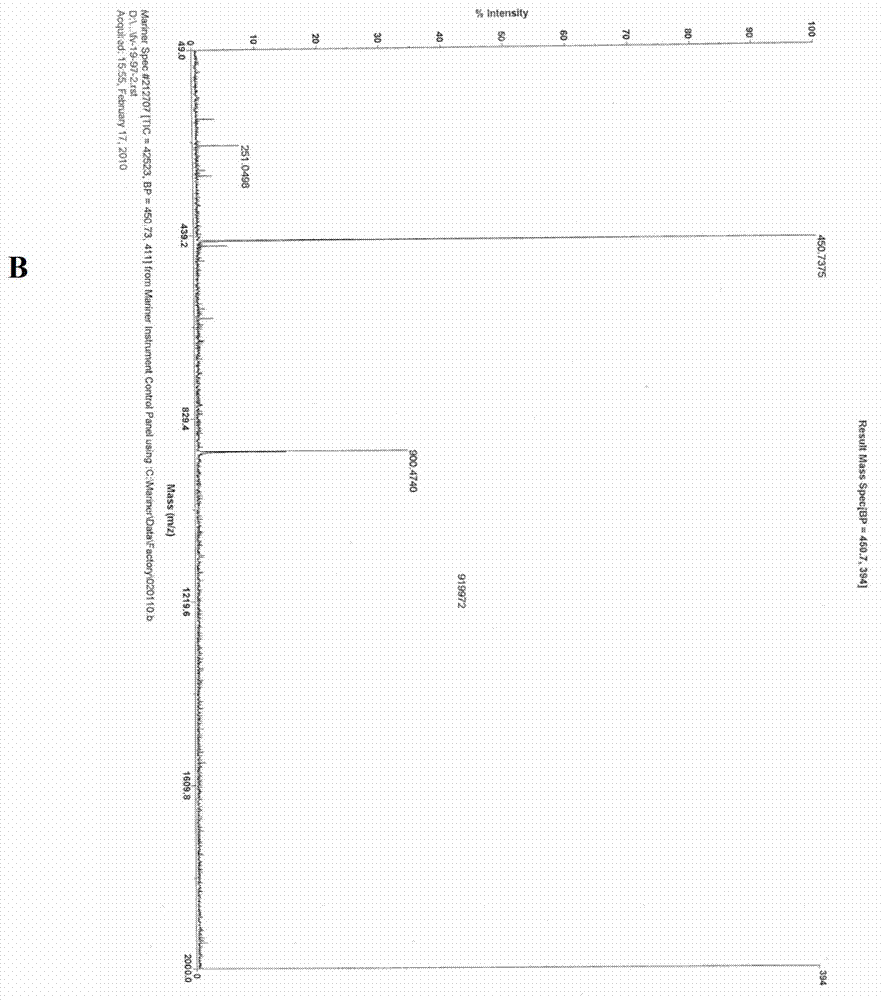
[0067] The synthetic steps of RGD-PEG-MPA are as follows figure 1 Shown, it is composed of the oligopeptide cyclo(-Arg-Gly-Asp-D-Phe-Lys-( figure 1 The amino group on Lys in A) is related to 8-amino-3,6-dioxa-octanoic acid ( figure 1 In B, the carboxyl group of PEG) is dehydrated and condensed to obtain RGD-PEG ( figure 1 C in C), then the amino group of RGD-PEG is combined with 3-Maleimidopropionic Acid ( MPA for short, figure 1 The carboxyl group in D) is dehydrated and condensed to obtain RGD-PEG-MPA ( figure 1 E in the tumor-targeting structural molecule). The specific synthesis of RGD-PEG-MPA was entrusted to Peptide International Company of the United States (with a confidentiality agreement signed). Its identification and purification as figure 2 A-C....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com