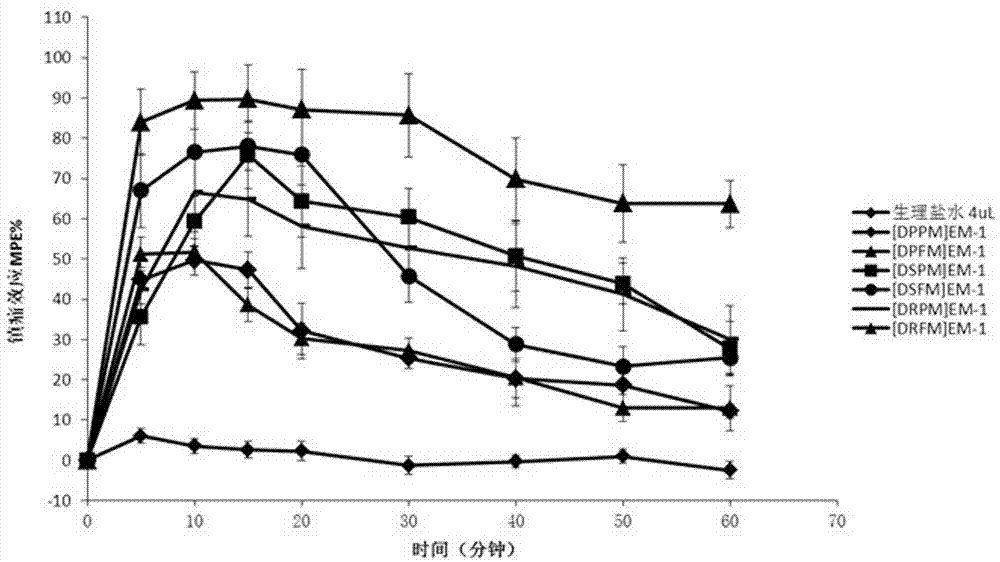
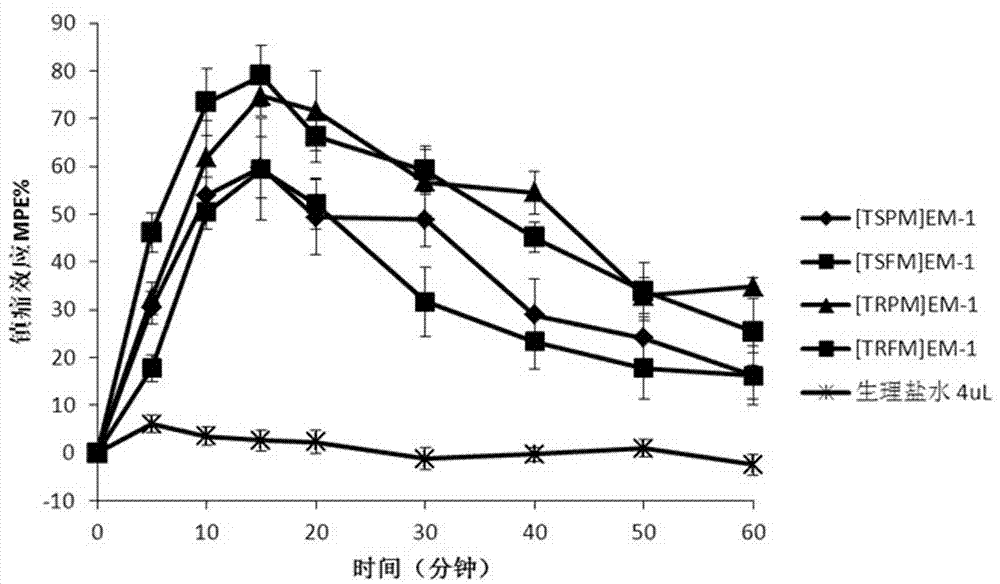
Synthesis and application of multi-site modified endomorphin-1 analogue
An endomorphin and multi-site technology, applied in the field of biomedicine, can solve the problems of short action time and poor enzymatic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Embodiment one, the synthesis of [DPPM]-EM-1
[0107] (1) Synthesis of N-tert-butoxycarbonyl-3-amino-2-methenyl-3-phenylpropionic acid amide
[0108] Dissolve 1 mole of N-tert-butoxycarbonyl-3-amino-2-methenyl-3-phenylpropionic acid in anhydrous dichloromethane, and add 4 moles of N,N-diisopropylethyl Baseamine, 1.45 moles of 1-hydroxybenzotriazole, 1.6 moles of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, stirred and dissolved fully, added 1.2 moles of ammonia water, and reacted at room temperature for 12 hours Add dichloromethane to dilute after the reaction is finished, wash three times with 5% citric acid solution, wash three times with saturated sodium bicarbonate, wash once with saturated sodium chloride, dry over anhydrous sodium sulfate, and distill under reduced pressure to obtain a white solid product——N- tert-butoxycarbonyl-3-amino-2-methenyl-3-phenylpropanoic acid; 90% yield.
[0109] (2) Synthesis of 3-amino-2-methenyl-3-phenylpropionic acid amide
[...
Embodiment 2
[0124] Embodiment two, the preparation of [DPFM]-EM-1
[0125] (1) Synthesis of N-tert-butoxycarbonyl-3-amino-2-methenyl-3-(2-furan)propionic acid amide
[0126] Dissolve 1 mole of N-tert-butoxycarbonyl-3-amino-2-methenyl-3-(2-furan)propionic acid in anhydrous dichloromethane, and add 4 moles of N,N-dichloromethane successively under stirring at 0°C Isopropylethylamine, 1.45 moles of 1-hydroxybenzotriazole, 1.6 moles of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, stirred and fully dissolved, then added 1.2 moles of ammonia water, React at room temperature for 12 hours; add dichloromethane to dilute after the reaction, wash three times with 5% citric acid solution, wash three times with saturated sodium bicarbonate, wash once with saturated sodium chloride, dry over anhydrous sodium sulfate, and distill under reduced pressure to obtain a white solid product - N-tert-butoxycarbonyl-3-amino-2-methenyl-3-(2-furan)propionic acid, yield 88%.
[0127] (2) Synthesis of 3-amino-2...
Embodiment 3
[0142] Embodiment three, the synthesis of [DSPM]-EM-1
[0143] (1) Synthesis of N-tert-butoxycarbonyl-3-amino-2-methenyl-3-phenylpropionic acid amide: same as Example 1.
[0144] (2) Synthesis of 3-amino-2-methenyl-3-phenylpropionic acid amide: same as Example 1.
[0145] (3) Synthesis of N-tert-butoxycarbonyl-tryptophan-3-amino-2-methenyl-3-phenylpropionic acid amide: same as Example 1.
[0146] (4) Synthesis of N-tert-butoxycarbonyl-2,6-dimethyltyrosine-(S)β-proline
[0147] Dissolve 1 mole of N-tert-butoxycarbonyl-2,6-dimethyltyrosine in anhydrous dichloromethane, add 4 moles of N,N-diisopropylethylamine successively under stirring at 0°C, 1.45 moles 1-Hydroxybenzotriazole, 1.6 moles of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, after fully dissolved by stirring, add 1.2 moles of (S) β-proline saturated carbonic acid Sodium hydrogen solution, react at room temperature for 12 hours, add dichloromethane to dilute after the reaction, wash three times with 5% citric aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com