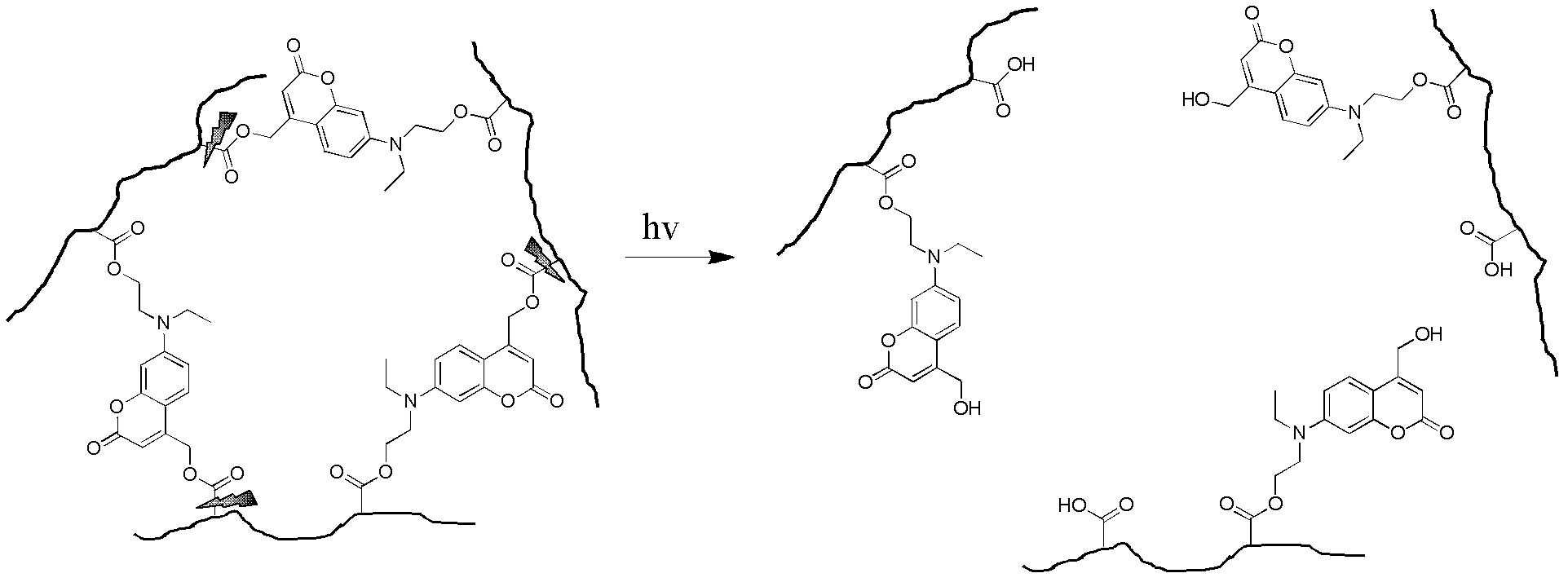
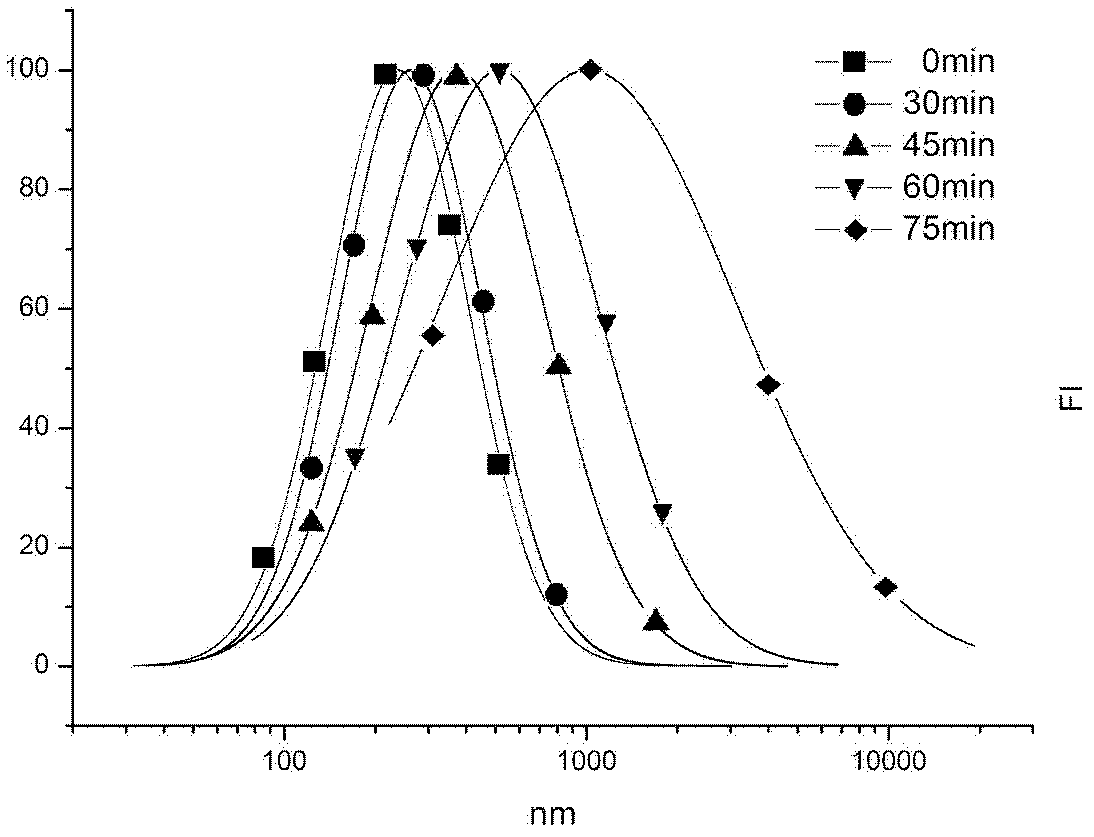
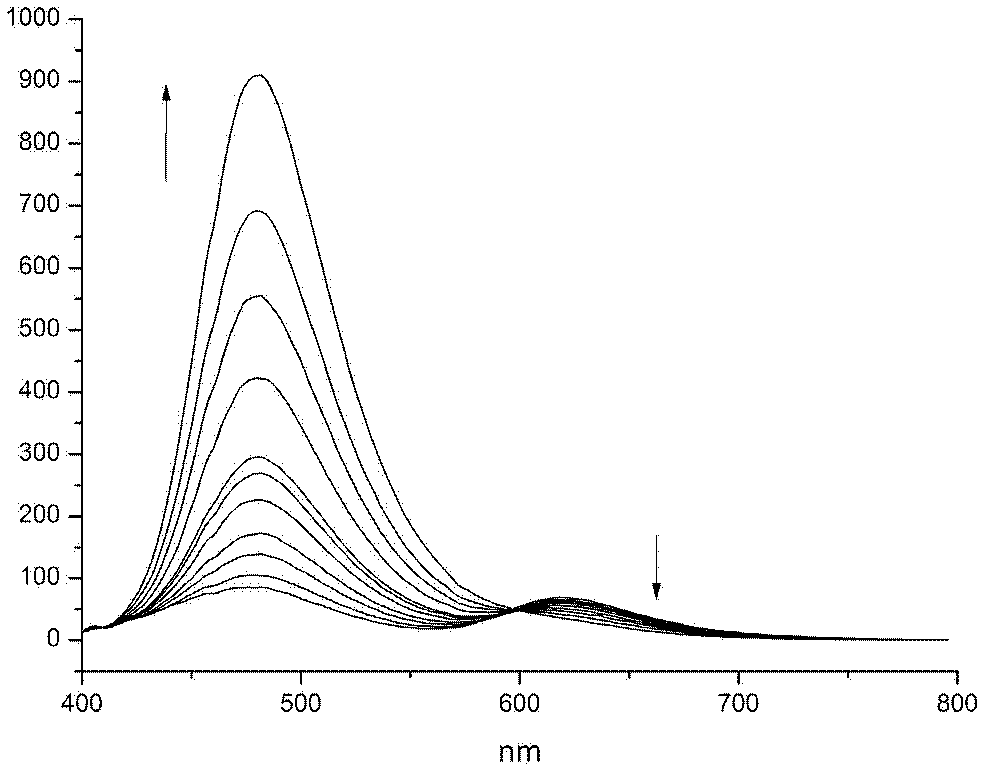
A photodegradable crosslinking agent and a preparation method and applications thereof
A technology of photodegradation and cross-linking agent, applied in the direction of organic chemistry, can solve the problems of limited application, impact on the environment and human body to be discussed, and achieve the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] When R 1 =COC(CH 3 )CH 2 , R 2 = H, R 3 =(CH 2 CH 2 ) m COC(CH 3 )CH 2 , when m=1 wherein, the preparation of crosslinking agent example A is as follows:
[0053]
[0054] Get 30 grams of resorcinol and 50 grams of sulfuric acid with a weight concentration of 95% and add them to a 100 milliliter round bottom flask, and add 45 grams of ethyl 4-chloroacetoacetate dropwise under an ice bath. After the dropwise addition, the reaction system was returned to room temperature, and the reaction was continued for 2 hours.
[0055] The reaction solution was added to ice water, and a large amount of white solid precipitated out. Filter and wash the solid to obtain compound 2.
[0056] Take 10 g of compound 2 and 1000 g of saturated potassium carbonate aqueous solution into a 2000 ml round bottom flask. Heating to reflux for 24 hours, cooling to room temperature, a white solid precipitated out. Filter and wash the solid to obtain compound 3.
[0057] 5 g of compoun...
Embodiment 2
[0062] When R 1 = H, R 2 = H, R 6 =(CH 2 CH 2 )y, where y=2, that is, polyol crosslinking agent compound B and when R 1 =COCHCH 2 , R 2 = H, R 6 =(CH 2 CH 2 ) y, when y=2, namely the preparation of crosslinking agent example C is as follows:
[0063]
[0064] Take 5 g of compound 3 and 5.57 g of 1,4-dibromobutane in 100 ml of acetone, add 7.19 g of potassium carbonate, and heat to reflux for 12 hours. Cool to room temperature, filter to remove the solid, distill off the solvent, and recrystallize from ethyl acetate to diol cross-linking agent, compound B.
[0065] 4 g of Compound B was dissolved in 50 g of dichloroethane, and 2.10 g of methacryloyl chloride was slowly added dropwise in an ice-water bath. After the dropwise addition, the reaction system was returned to room temperature, washed with water three times, the organic phase was evaporated, and recrystallized from ethyl acetate to obtain light yellow crosslinking agent C.
[0066] B's 1 H NMR spectral ...
Embodiment 4
[0071] Get 30 mg of crosslinking agent C and dissolve in 1 ml of DMSO: H O = 2: 1 mixed solvent, mix with 1 ml of aqueous solution containing 0.3 g of hydroxyethyl methacrylate and 10 mg of ammonium persulfate. Stir well and place in 3ml capsules. The reaction system was filled with nitrogen for 30 minutes to exclude oxygen. Add 100 micrograms of tetramethyldiethylamine and react at room temperature for 30 minutes to obtain a photoresponsive hydrogel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com