Nitrogen mustard phospholipid compound and preparation method thereof
A nitrogen mustard phospholipid and compound technology, which is applied in the field of nitrogen mustard phospholipid compounds and preparation, can solve the problems of short half-life, poor selectivity, high toxicity and the like, and achieve the effects of low toxic side effects and prolonged release half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
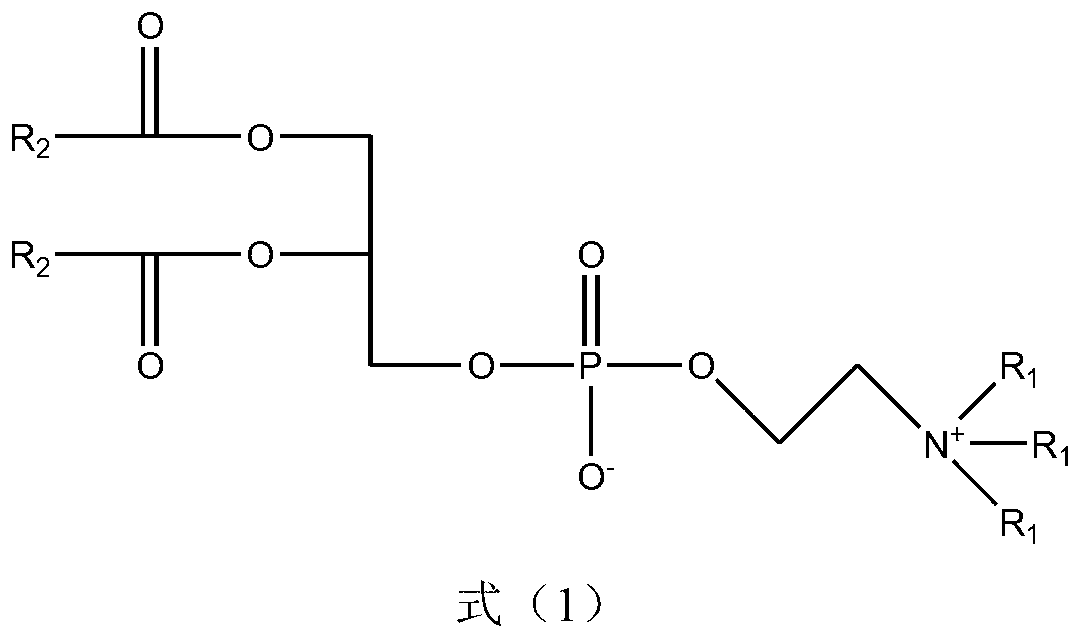
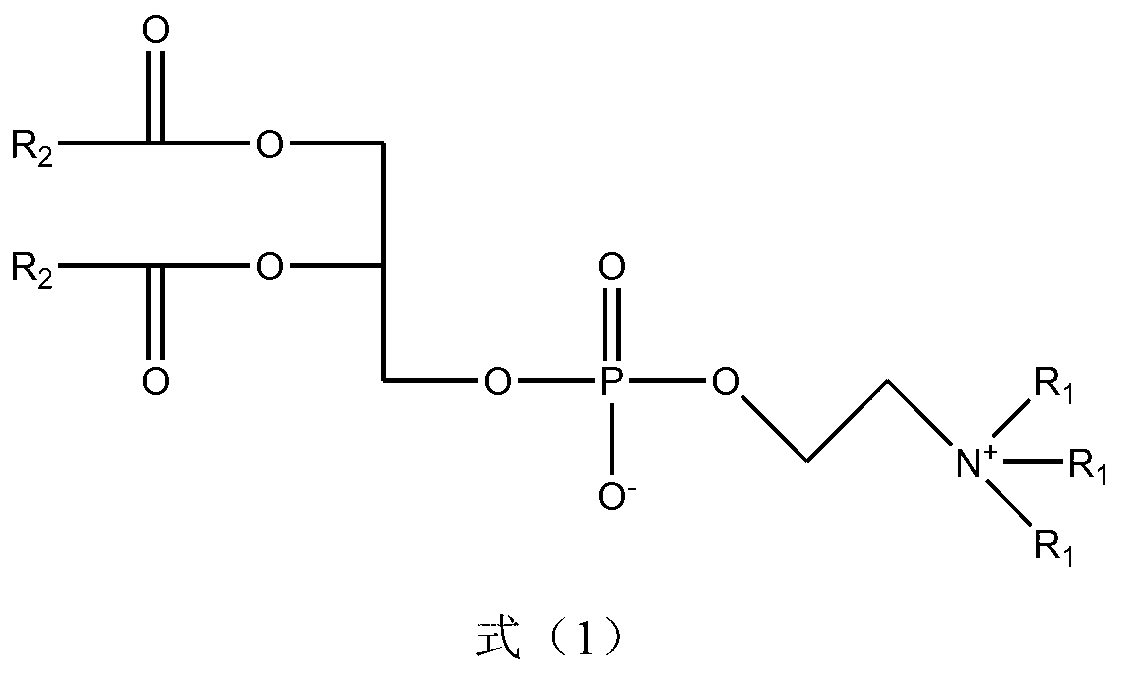
[0032] The preparation method of formula (1) proposed by the present invention uses nitrogen mustardic anhydride or nitrogen mustard acid chloride and glycerophospholipid derivatives as reaction reagents, and organic bases in organic solvents are used as catalysts to realize the reaction. The reaction formula is expressed as follows:
[0033]
[0034] R 1 Represents one or several groups such as hydrogen atom or hydrocarbon group, R 2 Representative (ClCH 2 CH 2 ) 2 NR 3 , R 3 Represents one or several groups such as hydrocarbon group, aryl group, aromatic hydrocarbon group, etc.
[0035] - "hydrocarbyl" is defined as C 1 -C 18 straight-chain or branched-chain alkanes or alkenes
[0036] - "Aromatic group" is defined as a hydrocarbyl group connected to an aryl group; the meaning of the hydrocarbyl group here is as defined above
[0037] In the preparation method of the compound of formula (1), the organic solvent is one or a mixture of dichloromethane, chloroform, ...
Embodiment 1
[0040] Preparation of compound I
[0041]
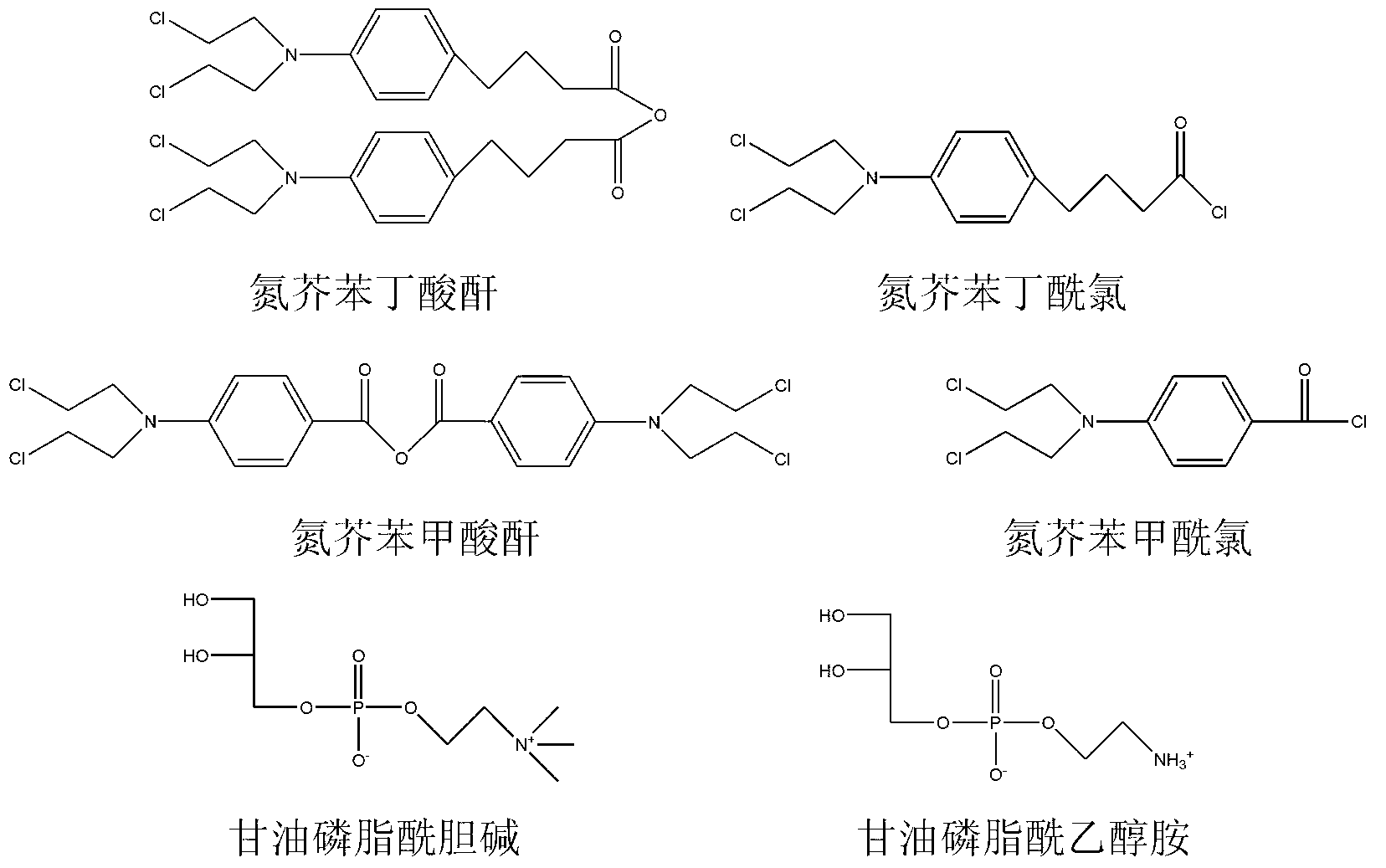
[0042] The synthetic route of compound I is as the above formula. Add nitrogen mustard benzoyl chloride (2mmol), glycerol phosphatidylcholine (1mmol), and 10ml of chloroform as solvent into the round bottom flask. After stirring, add 4-dimethylaminopyridine (DMAP ), heated for 24 hours. Thin-layer liquid chromatography showed that after the reaction was completed, the reaction system was washed three times with water, chloroform was removed by vacuum rotary evaporation, and a white or yellowish product was obtained by separation on a silica gel column with a yield of 80%.
Embodiment 2
[0044] Preparation of Compound II
[0045]
[0046] The synthetic route of compound II is as above formula. Add nitrogen mustard benzenebutyryl chloride (1mmol), glycerol phosphatidylcholine (1mmol) and 10ml of chloroform as solvent into a round bottom flask. After stirring, add DMAP and react at room temperature for 5 hours. Thin-layer liquid chromatography showed that after the reaction was completed, the reaction system was washed three times with water, chloroform was removed by vacuum rotary evaporation, and a white or yellowish product was obtained by separation on a silica gel column with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com