Preparation method of rivastigmine intermediate (R)-N-ethyl-N-methyl carbamic acid-3-(1-hydroxyethyl) phenyl ester
A technology of methyl carbamic acid and acetyl phenyl ester, which is applied in the field of preparation of rivastigmine intermediate-N-ethyl-N-methyl carbamic acid-3-phenyl ester, can solve the problem of unfavorable hydrogen source for environmental protection, Achieving high industrial application value, good selectivity, and cost-saving effects due to the problems of large catalyst consumption and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
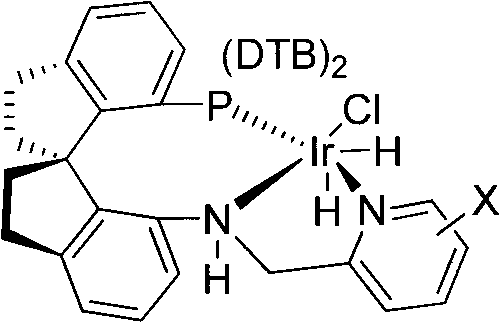
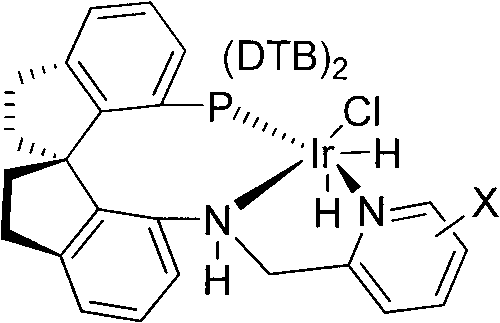
[0024] Embodiment 1: Preparation of (R)-N-ethyl-N-methylcarbamate-3-(1-hydroxyethyl)phenyl ester
[0025] Weigh (1.0mg, 0.001mmol) chiral catalyst (X is 3-methyl) and (224mg, 2.0mmol) potassium tert-butoxide into the reaction inner tube, put the reaction inner tube into the autoclave, and add to the reaction Add 30 mL of ethanol and (22.2 g, 100 mmol) N-ethyl-N-methylcarbamate-3-acetylphenyl ester into the inner tube, replace the gas in the kettle body with hydrogen, keep the hydrogen pressure at 0.2-10 MPa, and put the reaction The kettle was moved into an oil bath at 50°C to stir the reaction. After reacting for 9 hours, the hydrogen pressure no longer drops, and the heating and reaction are stopped. The reaction solution was concentrated. Add 100mL of water and 100mL of ethyl acetate to the system, and separate the layers. The aqueous phase was extracted twice with ethyl acetate (50 mL×2), the organic phases were combined, washed once with saturated brine, and dried over...
Embodiment 2
[0026] Embodiment 2: Preparation of (R)-N-ethyl-N-methylcarbamate-3-(1-hydroxyethyl)phenyl ester
[0027] Weigh (1.0mg, 0.001mmol) chiral catalyst (X is 6-ethyl) and (78mg, 2.0mmol) sodium amide into the reaction inner tube, put the reaction inner tube into the autoclave, and insert the reaction inner tube Add methanol 20mL and (11.1g, 50mmol) N-ethyl-N-methylcarbamate-3-acetylphenyl ester, replace the gas in the kettle body with hydrogen, keep the hydrogen pressure at 0.2-10MPa, and move the reaction kettle into The reaction was stirred in an oil bath at 50°C. After reacting for 8 hours, the hydrogen pressure no longer drops, and the heating and reaction are stopped. The reaction solution was concentrated. Add 80mL of water and 80mL of ethyl acetate to the system, and separate the layers. The aqueous phase was extracted twice with ethyl acetate (50 mL×2), the organic phases were combined, washed once with saturated brine, and dried over anhydrous sodium sulfate. Suction f...
Embodiment 3
[0028] Embodiment 3: Preparation of (R)-N-ethyl-N-methylcarbamate-3-(1-hydroxyethyl)phenyl ester
[0029] Weigh (1.9mg.0.002mmol) chiral catalyst (X is H) and (68mg, 1.0mmol) sodium ethoxide into the inner tube of the reaction, put the inner tube into the autoclave, and add tetrahydrofuran into the inner tube of the reaction 40mL and N-ethyl-N-methylcarbamate-3-acetylphenyl ester (44.3g, 200mmol), replace the gas in the kettle body with hydrogen, keep the hydrogen pressure at 0.2-10MPa, and move the reaction kettle into a 50°C The reaction was stirred in an oil bath. After 12 hours of reaction, the hydrogen pressure no longer drops, and the heating and reaction are stopped. The reaction solution was concentrated. Add 100mL of water and 100mL of ethyl acetate to the system, and separate the layers. The aqueous phase was extracted twice with ethyl acetate (100 mL×2), and the organic phases were combined, washed once with saturated brine, and dried over anhydrous sodium sulfat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com